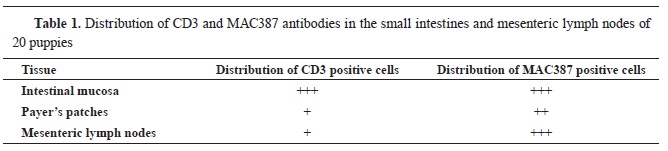
There is little data on the use of the immunohistochemical method in the observation of the T lymphocytes and macrophages distribution within the mesenteric lymph nodes in canine parvovirus (CPV) enteritis. The virus initially replicates in the systemic lymphoid tissue and causes highest changes in the small intestine. This current study aimed to demonstrate the CD3 and MAC387 antibodies detection and distribution in mesenteric lymph nodes and small intestines in dogs which had positive clinical, pathological, and histological findings of CPV. Twenty dogs that were clinically confirmed on CPV, had been autopsied following onset of death. Tissue samples (lymph nodes and small intestines) from each dog was processed for histology and immunohistochemistry utilizing hematoxylin-eosin and peroxidase/DAB (3,3′-diaminobenzidine) staining methods, respectively. The virus distribution was greatest in the tissue areas of the small intestine and the lymph nodes where the virus had made the most severe tissue damage. The distribution of macrophage was highest in the necrotic areas of the small intestine and the mesenteric lymph nodes. The amount of CD3 positive lymphocytes was greatly reduced in the mesenteric lymph nodes and the lymphoid tissue of the small intestine.
Canine parvoviral enteritis is a global disease caused by canine parvovirus (CPV) (
1). The virus is highly contagious and causes hemorrhagic gastroenteritis in adult and non-suppurative myocarditis in young dogs (
2, 3). There are three strains of the virus, namely CPV-2a, CPV-2b, and CPV-2c (
4). The disease is highly contagious with a significant mortality rate in young dogs and other carnivores. Its main pathological features are severe gastroenteritis and myocarditis (
2). The disease affects dogs of any breed, age, or sex, with puppies between 6 weeks and 6 months of age being more disposed to it (
5, 6, 8). The Parvoviridae are small non-enveloped, single-stranded DNA viruses (
6, 7, 9). The virus targets and replicates in rapidly dividing cells like the intestinal crypt epithelial cells, precursor cells in the bone marrow, and myocardiocytes, resulting in cell death (
6, 10). Immunodeficiency due to low colostrum antibodies, late vaccination, as well as ineffective responses to vaccinations, make puppies susceptible to this virus (
11). The virus transmission between dogs is mostly indirect, by fecal-oral route (
12, 13), but it can also be transmitted directly (
14). The clinical manifestation of the infection can be affected by pre-existing infections, age, immune status, dose, and virulence of the virus (
15). The parvoviral enteritis (PVE) is treated by symptomatic and supportive therapy with antiemetic, antibiotic, fluid, and antiviral treatments. (
5, 16). The virus replicates initially in the lymphoid tissue of the oropharynx, thymus, and mesenteric lymph nodes. It spreads through the blood to the small intestine 3–4 days after the infection (
6, 17, 18, 19). The mesenteric lymph nodes become edematous and congested. The changes in the small intestine take place mainly in the jejunum and ileum, in some cases, in the duodenum as well. In the small intestine, the virus causes enteritis characterized by thickening and discoloration of the intestinal wall with mucosal and serosal hemorrhage. The intestinal content is thin and bloody. Histologically, there is lymphoid depletion and necrosis in the systemic lymphoid tissue. There is necrosis of the crypt epithelium in the small intestine. The crypts may be distended and usually necrotic (
20, 21, 22, 23, 24).
The current study aimed to make an immunohistochemical detection and distribution of CD3-positive lymphocytes and MAC387-positive macrophage in the lymphoid tissue and the small intestine of dogs which had positive clinical, pathological, and histological findings of CPV.
MATERIAL AND METHODSAnimalsThis study was conducted in the span of a two-year period. It included twenty dogs at age between 2 and 12 months. They were clinically diagnosed to be positive on PVE with history of anorexia vomiting, and bloody diarrhea with specific odor. The dogs were treated with symptomatic therapy: fluid therapy, intravenous antibiotic (Ceftriaxon), and antiemetic (Metoclopramid).
Clinical and necropsy examinationsAll dogs were clinically examined for body condition, body temperature, heart and respiratory rate, appetite, and signs of vomiting. Coprological examination was performed for helminths, protozoa, and canine parvovirus. The serological confirmation of canine parvovirus was performed with “Antigen rapid test kit for CPV” (BIONOTE). The postmortem examination was conducted with verbal consent by their owners within 24 hours after the animal’s death. Small intestine and mesenteric lymph nodes were sampled from each animal.
Histological and immunohistochemical stainingTissue samples from the mesenteric lymph nodes and the small intestine were taken and fixed in 10% neutral buffered formalin for about 48-72 hours. For the histological examination, the tissues were cut in 3-4 μm sections, stained with hematoxylin and counterstained with eosin. For the immunohistochemical investigation, CD3 and MAC387 monoclonal antibodies were used with DAKO Envision kit based on the Peroxidase/DAB method. This was done in order to determine the impact of the CPV antigen concentration and distribution on the lymphocyte and macrophage distribution within the same tissue samples. For the immunohistochemical staining, 5 μm thick sections were placed on to positively charged slides. After the deparaffinization of the tissue sections, the antigen retrieval was performed by citrate buffer pH 6.0 and heat induction in microwave at 500W for 20 min. The Endogenous peroxidase was quenched by the slides’ incubation in 3% H
2O
2 for 20 min. The slides were incubated overnight in a humidified chamber at 4 °C with CD3 and MAC387 monoclonal antibody, respectively at a dilution of 1:200 in PBS. Secondary antibody, labelled horseradish peroxidase (HRP) and 3, 3 diaminobenzidine tetrahydrochloride (DAB) were added respectively following washing with PBS. Finally, the sections were counterstained with hematoxylin, dried and covered with glass cover slip. Normal rabbit sera were used for the treatment of control tissue sections.
RESULTSClinical examinationOn examination, all of the dogs showed similar clinical signs. The frequent vomiting and bloody diarrhea had led to general weakness and dehydration. The diarrhea had a specific foul odor characteristic for PVE. Their temperature ranged from 38.5 °C to 40.5 °C. The coprological examination for parasites was negative, however, the parvovirus antigen test was positive. The hematological test showed severe anemia and leucopenia.
Necropsy findingsAll carcasses were dehydrated and cachectic. The mucous membranes of the mouth and eyes were anemic and the anus was smeared with bloody feces. The heart was enlarged. The lungs, liver, and spleen were congested with visible hemorrhages. The stomach mucosa was hyperemic, however, the main finding in all carcasses was hemorrhagic enteritis of the small intestine, most emphasized in the distal jejunum and ileum characterized by reddening and thickening of the mucosa which was covered by diphtheroid membrane. The intestinal lumen was usually empty, or contained a small quantity of reddish fluid. The Payer’s patches and the mesenteric lymph nodes were enlarged and hemorrhagic. When cut, the parenchyma of the mesenteric lymph nodes was red.
Microscopic findingsTwelve out of twenty dogs had hemorrhagic enteritis, and the rest had severe catarrhal enteritis of the small intestine with most severe lesions in the ileum and duodenum. In three dogs, the lesions included the entire small intestine. The intestinal glands were completely destroyed. Microscopically, the damage of the intestinal villi ranged from lack of enterocytes to complete villi atrophy with an epithelial cell debris inside the intestinal lumen. There were areas of crypt hyperplasia with lymphocyte, macrophage, and a mild degree of neutrophilic infiltration, while other areas had multifocal crypt necrosis. The necrosis of crypt epithelial cells was widespread. The lymphocyte infiltration was located mainly in the mucosa of the small intestine. There was hemorrhage and necrosis of lymphoid follicles in the intestinal lymphoid nodules and Peyer’s patches with visible lymphoid depletion, mainly in their cortex. The paracortex areas had multiple macrophages. These findings were also seen in the mesenteric lymph nodes where the lymphoid depletion mostly affected the germinal centers.
The paracortex had abundant macrophages filled with cell debris.
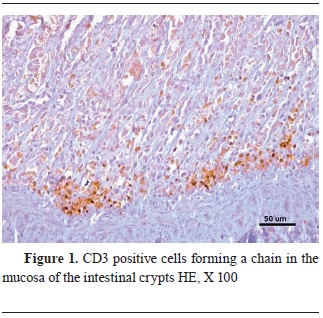
ImmunohistochemistryThe virus antigen was highly present in the lymphoid tissue of the mesenteric lymph nodes and the intestine, more specifically inside the lymphocytes. The jejunum and ileum were also significantly marked by the CPV antibodies, concentrated in the remaining epithelial cells, in the dead cells of the necrotic crypts, and in the lymphoid tissue. They were not detected in the duodenum and colon. There was CD3 and MAC387 positive cell infiltration within the areas of crypt hyperplasia. The lymphocyte infiltration was located mainly in the mucosa of the small intestine, concentrated in a chain formation (
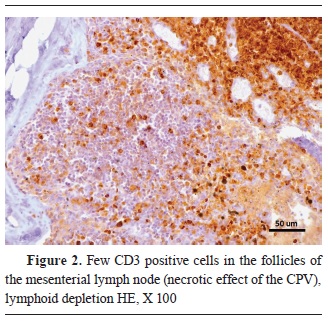
Fig. 1). The areas where the viral pathogen was present in the greatest amount were also the areas with the lowest CD3 signal. Inside the lymphoid follicles, the immunohistochemical staining for lymphocytes was much weaker, suggesting a severe lymphoid depletion. That was particularly noticeable in the germinal centers of the mesenteric lymph nodes (
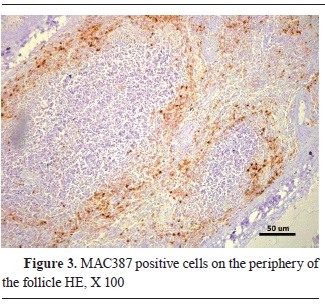
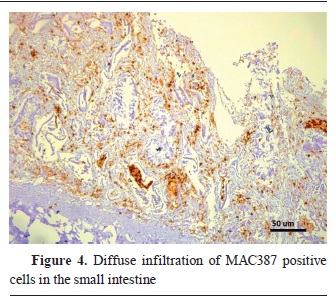
Fig. 2). On the other hand, the macrophage antibodies were mostly visible in the paracortex of the lymph follicles, and with a diffuse infiltration in the intestinal villi (
Fig. 3 and
4). The distribution of CD3 and MAC387 antibodies is presented in
Table 1.
 DISCUSSION
DISCUSSIONCanine parvovirus (CPV) causes high mortality in dogs. It is manifested in cardiac and enteric form in young pups and in adult dogs, respectively (
3). None of the dogs included in the current study had the cardiac form. They were all affected by the enteric form. The clinical signs of CPV infection include vomiting, diarrhea, dehydration, fever, depression, and leukopenia, however, they are not pathognomonic for CPV as they are found in a range of other diseases. That is why authors suggest that a definitive diagnosis for CPV should include serology, necropsy with histopathology, and immunohistochemistry (
7, 25, 26). Necropsy findings in animals suffering from parvoviral enteritis, especially those with fatal outcomes like those in the current study, generally include necrohemorrhagic enteritis with extensive necrosis of intestinal gland epithelium, lymphoid depletion (lymphopenia), and lymphoid necrosis (
20, 21, 22, 27). According to Meunier et al. (
19, 22), the virus infection in the mesenteric lymph nodes precedes that of the intestinal epithelial and intestinal lymphoid tissue infection. The immunohistochemical study showed a significant lymphoid depletion. The lymph nodes were full of PCV antibodies but lacked the normal CD3 expression. These areas were infiltrated by MAC387 positive macrophage. The current study confirmed those findings and demonstrated that the places of initial virus replication, the lymphoid tissue in the mesenteric lymph nodes and the lymphoid follicles of the small intestine (
6, 18, 19), are most highly affected and damaged by the virus replication. The enterocytes and the T- lymphocytes are rapidly dividing cells (
9, 10). The current study demonstrated that the enterocytes and the T lymphocytes were the target cells of the CPV visualized by immunohistochemistry. This was in agreement with other reports which stated that the virus replicates in the rapidly dividing cells of the intestine and the systemic lymphoid tissue (
1, 2, 3, 4, 5). In a previous study, we demonstrated a strong PCV infiltration in the damaged areas of the lymph follicles (
11). The same areas were depleted of T lymphocytes showing a weak CD3 positive signaling.
CONCLUSIONThe enterocytes and the T lymphocytes were the target cells of the CPV. The lymphoid tissue in the mesenteric lymph nodes and the lymphoid follicles of the small intestine were most highly affected by the CPV. There were almost no lymphocytes in the mesenteric lymph nodes. The mucosal layer of the intestine was most highly infiltrated with the CPV antibodies. The lymph nodes were also highly infiltrated but lacked the normal CD3 expression. MAC387 positive macrophage infiltration was also observed in these layers. They were located in the paracortex of the lymph follicles.
CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSThis work was supported by the project “Aplication of the immunohistochemical method in the diagnostic of virus diseases in domestic animals” funded by the Faculty of Veterinary Medicine-Skopje, Ss. Cyril and Methodius University in Skopje (FVMS-IPR-03).
AUTHORS’ CONTRIBUTIONIG and TN wrote the article. SB and TN collected the samples. SB, TN, AJ, TR and NA analyzed the samples. All authors approved the final version of the study.

 10.2478/macvetrev-2024-0014
10.2478/macvetrev-2024-0014




