The present study aimed to determinate the effect of external progesterone (P4) supplementation on luteolysis in cows under heat stress. Forty-eight (n=48) dairy cows in the period from July September 2018 were part of and at day 35±3 postpartum scored for BSC, synchronized using PG-3-G + Ovsynch protocol and randomly allocated into two treatments: PRID group (n=27) treated with external P4 device between G1 and PGF2α and CON group (n=21) left without treatment. Collection of blood samples to assess P4 concentrations was done at Pre-PG, at G1, at PGF2α, at 72 h after PGF2α (at timed artificial insemination TAI) and at d 21 after TAI. The pregnancy diagnosis was done at d 21 and d 30 after TAI by ultrasound. The average temperature humidity index (THI) was 79.5±0.6. At G1, the P4 was significantly lower in the PRID group (1.84±0.99 ng/mL) in comparison to the CON group (2.97±1.82 ng/mL). In contrast, at PGF2α, there was a tendency (p=0.09) of increased P4 concentration in PRID group compared with the CON group (4.26±1.68 and 3.74±2.39 ng/mL), respectively. At TAI, more PRID cows (p=0.0001) had a lower P4 (0.06±0.03 ng/mL), in comparison to CON (1.28±2.41 ng/mL). At d 21 and d 30 after TAI, more PRID cows were predicted and diagnosed pregnant (16/27 or 59.25% and 13/27 or 48.14%) compared with the CON group (11/21 or 52.38% and 8/21 or 38.08%) respectively, but without any significant differences. Supplementation of the P4 during the Ovsynch protocol increases the P4 before TAI and reduces the incomplete luteolysis in heat stressed dairy cows.
In the last few decades, heat stress has become a global problem that threatens the health and welfare of animals. The ambient temperature optimal for good animal health and welfare generally ranges between 16 to 25 °C. The upper critical point of this range marks the onset of heat stress. Heat stress adversely affects fertility of dairy cattle and compromises their reproductive function through reduced expression of estrus, disruption of follicular and oocyte function, as well as increased embryonic mortality (
1). In addition, cows chronically exposed to heat stress have a reduced progesterone (P4) concentration (
2) leading to decreased fertility due to its importance on pregnancy maintenance. Therefore, many hormonal therapy strategies have been implemented in order to increase the circulating P4 concentration that might positively affect fertility in heat-stressed dairy cows. In this regard, López-Gatius et al. (
3) demonstrated an improved fertility after treating the cows with GnRH after AI, due to an increased number of accessory CLs and thus elevated P4 after AI. Similarly, Mendonca et al. (
4) have shown an improved fertility in heat stressed dairy cows after treatment with GnRH at day 5 after AI. In addition, Denicol et al. (
5), have demonstrated that follicles that grew under elevated P4 concentration (second follicular wave) were more fertile in comparison with follicles growing under low P4 concentration (first follicular wave). Cumulatively, all of these studies have shown the importance of elevated P4 concentration before and after AI that might positively affect fertility. On the other hand, increased circulating P4 concentration near AI can result in a reduced fertility due to an inadequate luteolysis. The latter has been clearly shown in numerous studies using timed AI programs (
6). Indeed, up to 30% of cows submitted either to an Ovsynch 56 protocol (G1–7 days – PGF
2α – 56 h – G2 – 16 h – TAI) (
7) or to a single PGF
2α estrussynchronization protocol (
8) had an incomplete luteolysis. Increased concentration of P4 near AI (>0.4 ng/mL), due to incomplete luteolysis, reduces fertility (
9) because elevated P4 at the time of G2 reduces the magnitude of the GnRH-induced LH surge (
10). Thus, the failure of complete CL regression limits the achievement of maximal P/AI (pregnancy per AI) (
11). Several strategies have been implemented to prevent incomplete luteal regression: by either increasing the dose of PGF
2α (
12) or the frequency of PGF
2α treatment (
13), or lengthening the time from G1 to PGF
2α for 1 day (Ovsynch 8) (
8, 14) during the Ovsynch protocol. However, the results were inconsistent.
We hypothesized that supplementation of exogenous progesterone will increase the P4 level during the Ovsynch protocol and consequently might reduce the incomplete luteal regression in heat stressed dairy cows. Therefore, the objective was to determine the effect of exogenous P4 on luteolysis in dairy cows that are under heat stress.
MATERIAL AND METHODSAnimals and synchronization protocolForty-eight (n=48) Holstein dairy cows from one herd during July-September 2018 were enrolled in the study. Cows were housed in free-stall barn, fed a total mixed ration (TMR) twice daily to meet or exceed production of on average 30 L of milk per day. Cows were milked twice daily on a 12 hours interval and had free access to water. All cows at day 35±3 after parturition, were evaluated for body condition (BCS, 1=emaciated and 5=obese) (
15) and pre-synchronized using PG-3-G protocol as described previously (
16). Briefly, the protocol consisted of injection of PGF
2α (Pre-PG; PGF Veyx Forte, Veyx-Pharma GmbH, Germany) followed by injection of GnRH (Pre-GnRH; Receptal, MSD Animal Health, GMBH, Germany) 3 days later. Seven days after the Pre-GnRH injection, an Ovsynch 56 protocol was initiated (GnRH1 (G1)– 7 days – PGF
2α – 56 h – (GnRH2 (G2) – 16 h – TAI). Cows were allocated randomly into two treatments: 1) PRID group (n=27), treated with external P4 (PRID-progesterone intravaginal releasing device, CEVA, Sante Animale, France) inserted at G1 and withdrawal at PGF
2α, and 2) CON group (n=21) left without any additional treatment.
Ultrasonographic examination, blood collection, cyclic status, luteolysis, progesterone analysis and pregnancy diagnosis
B-mode scanner (Mindray DP 50, Soma Technology Inc. USA) equipped with a 7.5 MHz rectal linear probe was used to map ovarian structures at G1 and at d 21 after TAI. Collection of blood samples to evaluate the P4 concentrations was done at Pre-PG, at G1, at PGF
2α, at TAI, and at d 21 after TAI from the coccygeal vein into evacuated tubes (BD Vacutainer® Plymouth, UK). The samples were refrigerated at +8 °C and transported to the lab at the Faculty of Veterinary Medicine–Skopje, North Macedonia within 3 h after collection. The sera were collected after centrifuging (1,000 x g for 15 min), and stored at -20 °C until P4 evaluation. The P4 evaluation was done on an Immuno-scan BDLS reader using commercially available kit (HUMAN, Progesterone ELISA Test–GMBH, Germany). The intra- and inter-assay CV were 4.2% and 7.8%, respectively. The P4 concentrations collected at Pre-PG and at G1 were used to determine the cyclic status of the cows. When both samples contained P4 level <1 ng/mL, the cows were classified as acyclic. On contrary, when P4 concentrations were ≥1 ng/mL either at Pre-PG or at G1, the cows were classified as cyclic. Incomplete luteal regression was defined to occur when the P4 level was >1 ng/mL at PGF
2α and >0.4 ng/mL at TAI, respectively (
14). Although, the pregnancy rate was not the primary focus of the study due to the small number of cows included, the ultrasound pregnancy diagnosis was done at d 21 and d 30 after TAI. Cows that had no CL and had P4 <0.5 ng/mL on d 21 after TAI, were predicted to be non-pregnant (
17). If there was one CL >25 mm with a follicle <13 mm, and P4 >2 ng/mL, the cows were predicted pregnant (
17). Positive pregnancy diagnosis at d 30 was declared when an embryo with a visible heartbeat was observed. In addition, cows were further clustered according to their BCS (<2.75 and ≥2.75).
Statistical analysisT-test and Duncan multiple range test were performed to compare the data between PRID and CON. Correlations analysis was performed for BCS and P4 for PRID and CON groups. Values were presented as means ±standard deviation.
Temperature and relative humidity measurements:
The data for Relative humidity (RH) and average daily temperature (T) values were collected from the nearest meteorological station and the temperature-humidity index (THI) was calculated as THI = (1.8 x T + 32) – ((0.55 - 0.0055 x RH) x (1.8 x T - 26)) (
18).
RESULTS
The average THI during the experiment was 79.5±0.6, showing that cows were under heat stress condition. Overall, all cows were classified as cyclic according to the P4 concentration (P4 >1 ng/mL) at PRE-PG and G1.
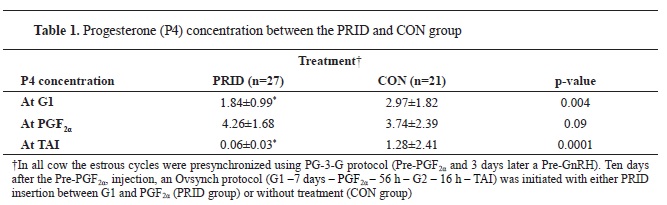
At G1, the P4 concentrations was lower in the PRID group (1.84±0.99 ng/mL) in comparison to the CON groups (2.97±1.82 ng/mL, p=0.004). In contrast, at PGF
2a, the PRID group of cows tended to have (p=0.09) an increased P4 concentration compared with the CON group (4.26±1.68 and 3.74±2.39 ng/mL, respectively). In addition, at TAI, the P4 concentration differed between the treatments (p=0.0001), with more PRID cows having a lower P4 concentration (0.06±0.03 ng/mL) compared with the CON group (1.28±2.41 ng/mL), implying that more PRID cows underwent a complete luteolysis in comparison with the CON group (
Table 1). At d 21 no differences (p=0.54) were detected in the P4 concentration between the treatments.
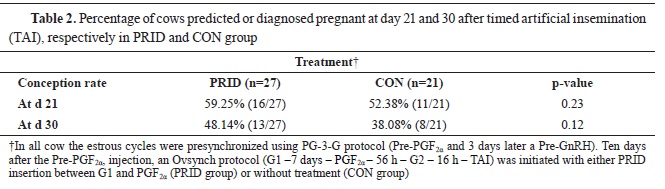
At d 21 after TAI, more PRID cows were predicted pregnant (16/27 or 59.25%) compared with the CON group (11/21 or 52.38%) but the differences were not significant. Similarly, at d 30 after TAI, more PRID cows were diagnosed pregnant (13/27 or 48.14%) compared with CON group of cows (8/21 or 38.08%,
Table 2). It was assumed that cows that were diagnosed pregnant at d 21 but non-pregnant at d 30, had experienced late embryonic mortality.
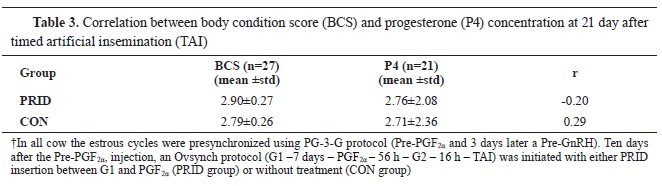
When cows were additionally clustered according to their BCS and correlated with the P4 concentration at G1, at PGF
2α, and at d 21 after TAI, no differences were detected (
Table 3). Similarly, no differences were observed in the pregnancy rates at d 30 among the cows with the same BCS between the PRID and the CON group of cows.
DISCUSSIONThe present study aimed to observe whether supplementation of exogenous P4 concentration during the Ovsynch protocol will reduce the percentage of cows with incomplete luteal regression in heat-stressed dairy cows. The results have shown that higher number of PRID-treated cows underwent a complete luteal regression compared to the not-treated CON group of cows with a single PGF
2α application in the Ovsynch protocol. In addition, our results showed that PRID treatment in heat-stressed cows is sufficient to increase the P4 level at the time preceding PGF
2α application. The latter is in agreement with the results observed by Bisinotto and Santos (
19), in which single insert delivers sufficient P4 by incrementing plasma concentrations during the development of the ovulatory follicle (near the time preceding PGF
2α application). Similarly, greater P4 concentrations at the time of PGF
2α were associated with greater probability of luteolysis after PGF
2α treatment (
20) as shown in the current study. Cumulatively, increased P4 concentration during the follicular growth accompanied with completed luteolysis after PGF
2α treatment and ovulatory success after G2 were associated with greater pregnancy rate (
21). Cows that were induced to ovulate the dominant follicle of the second follicular wave (high P4) have an increased pregnancy rate compared with the cows that were induced to ovulate the dominant follicle of the first follicular wave (low P4) (
5). Indeed, we have shown that PRID treated cows have an increased pregnancy rate at d 30 compared with CON group of cows. Although, the pregnancy rates were not the primary focus of the present study as a small number of cows were included in the study. Nevertheless, our results showed a 10% increase in pregnancy rate in PRID treated cows compared with CON group of cows. Xu et al. (
22) reported similar results where cows observed in estrus and inseminated after treatment with PGF
2α during early diestrus (d 5 to 9, low P4) had lower pregnancy rates compared with cows inseminated in estrus after PGF
2α treatment given on or after d 10 of the estrous cycle (high P4) (
22).
Based on the ultrasound examination and P4 level, a higher percentage of cows (more than 50%) were predicted for pregnancy at d 21 in both groups. Nevertheless, more cows in CON group at d 30 experienced an embryonic mortality compared with the PRID group of cows. Similarly, Cunha et al. (
23) observed an increase in pregnancy loss between d 29 and d 57 when cows had low P4 (14.3% loss) vs high P4 (6.8% loss) during growth of the follicular wave that produces the ovulatory follicle before AI. Nevertheless, the exact underlying physiological mechanisms of increased pregnancy loss in heat-stressed cows remain unclear. We are speculating that a partial luteal regression after single PGF
2α injection, as shown in the present study, elevate the P4 concentration (near the AI) that might increase the total α-inhibin production by the cumulus–oocyte complex, which may reduce the embryo development after cleavage (
24) and thus, increase the pregnancy loss. In addition, elevated P4 concentration might affect the uterine blood flow and thus functionality of the uterus that could results in reduced embryo development and increased pregnancy loss (
25).
The BCS was not related either with the luteolysis or with the pregnancy rate during the heat stress. These results do not corroborate with the results from our recent study (
26), where we have found that thinner cows (BCS<2.75) have greater failure in luteolysis in comparison to the cows with BCS≥2.75. It is unclear how body condition might influence luteolysis thus future studies need to be conducted to evaluate the aforementioned relationship.
CONCLUSIONSupplementation of the exogenous P4 during the standard Ovsynch protocol increases the P4 concentration before TAI and reduces the incomplete luteal regression in heat stressed dairy cows. In addition, elevated P4 concentrations before TAI increases the pregnancy rates and reduces the pregnancy loss in heat-stressed cows. Nevertheless, in order to confirm our findings, further studies are warranted, including a larger number of cows.
CONFLICT OF INTERESTSThe authors declare that they have no known conflict of interest in the conduction of the current study.
ACKNOWLEDGMENTSWe would like to acknowledge ZK Pelagonija Dairy Farm (Bitola, R.M) for allowing us the use of their facilities.
AUTHORS CONTRIBUTIONTD, MS, BS planed the study design. BA and TD performed the ultrasonographic examination of the cows. SV, KI and JG performed the proofreading. MN have run the statistical analysis. BS, TD and BA have synchronized the cows. BA and BS collected the blood samples.

 10.2478/macvetrev-2024-0015
10.2478/macvetrev-2024-0015


