Hyperadrenocorticism (HAC) in dogs is routinely treated with trilostane single-dose (CTG) which is reported to cause adverse reactions. The current retrospective study of several dogs with terminal stage of HAC aimed to compare the clinical, hematological, and biochemical effects of trilostane titration-dose treatment (TTG) with the single-dose treatment (CTG). All clinical cases (n=7) were confirmed on HAC by anamnestic, clinical, hematology, biochemistry, and low-dose dexamethasone suppression test findings, indicative for Cushing’s disease. Two cases were treated with CTG (2.2-6.7 mg/kg, single dose daily) and their treatment was discontinued on the second week due to adverse reactions. The TTG cases were treated for up to 12 weeks (0.5 mg/kg once daily for 7 days, and then with 0.5 mg/kg twice daily for 7 days). Blood samples and clinical checks were performed on 0., 4., and 12. weeks of the treatment. Hemoglobin was non-significantly higher in TTG at 12 weeks. Alanine transaminase was significantly lower in the TTG cases on the 12. week of the treatment (78.04±15.37 U/L) compared to the 0-week (137.81±24.03 U/L), and 4-week samples (131.92±23.36 U/L). No significant differences were observed with the CTG cases. Alkaline phosphatase was significantly lower on 12-week samples in TTG (251.02±93.06) compared to the 4-week (567.94±283.93 U/L), and 0-week samples (1,341.84 U/L). In conclusion, TTG has indicated to have significantly higher tendency to decrease alanine transaminase and alkaline phosphatase, alleviating the negative effects on the liver. The clinical findings were more adverse for the CTG.
Hyperadrenocorticism (HAC) or Cushing’s disease in dogs is one of the most commonly diagnosed endocrine diseases, caused by excessive secretion of adrenocorticotropic hormone, usually by pituitary adenoma (pituitary dependent) (
1). HAC is a clinical syndrome characterized by abnormally high concentrations of circulating glucocorticoids. Cortisol may exert various effects on tissues depending on the serum concentration. Clinical manifestation of HAC in dogs includes: polydipsia, polyuria, polyphagia, pendulous abdomen, muscle weakness, alopecia, infertility, etc., and are mainly caused by the glycogenic, immunosuppressive, anti-inflammatory, protein-catabolic, and lipolytic effects of the cortisol (
1). Laboratory findings usually reveal lymphopenia and eosinopenia which are most likely steroid-induced, elevated alanine transaminase (ALT) due to liver damage, increased alkaline phosphatase (ALP) due to steroid-induced isoenzymes, mild to moderate increases in the cholesterol and triglyceride concentrations and mild fasting hyperglycemia (
2, 3).
Screening tests designed to diagnose HAC include: the ACTH (ACTH) stimulation test, low-dose dexamethasone suppression test (LDDST), and the urinary cortisol:creatinine ratio. These methods can give false-negative and false-positive test results and can be used in dogs in which HAC is primarily diagnosed on the basis of historical and clinical findings (
4). LDDST can help in distinguishing pituitary-dependent HAC from adrenocortical tumor. If the suppression is <50% from the baseline value, it confirms the HAC as pituitary-dependent (
5).
Trilostane is an active synthetic steroid analog that inhibits 3-beta-hydroxysteroid dehydrogenase enzyme responsible for converting pregnenolone to progesterone. It is frequently used in the treatment of pituitary-dependent HAC or in the treatment of HAC from adrenocortical tumor. It is not cytotoxic and does not damage the adrenal cortex (
6). Numerous clinical studies have confirmed the efficacy of the trilostane HAC-treatment (
7, 8, 9). The recommended dose treatment (3-6 mg/kg per day,
per os) in the terminal stage of the disease can cause rapid decrease of the cortisol serum concentration, thus developing pituitary macroadenoma with neurological symptoms. The most frequently reported adverse effects are lethargy, depression, anorexia, vomiting, diarrhea, and weakness, which sometimes can be severe (
8, 10). A dual dosage was first reported in 2006, given twice daily, with initial dose from 1.25 to 2.75 mg/kg/12h (
11, 12). Following the success in the treatment and the clinical response, it has been frequently practiced ever since.
Considering the positive clinical reports and the incidence of serious adverse effects caused by trilostane treatment, we hypothesized that the adjustment of the trilostane dose can reduce the side effects in dogs with terminal stage of HAC and various comorbidities. The current retrospective study aimed to investigate the hematological, biochemical, and clinical variations of several dog cases diagnosed with terminal stage of HAC and various comorbidities, treated with the recommended trilostane dose and the adjusted titration trilostane dose.
MATERIAL AND METHODSThe current retrospective study included patient’s data from the University Veterinary Hospital in Skopje with verbal consent by the owners. The dogs (n=7) were diagnosed with terminal stage HAC by clinical and laboratory findings. The LDDST confirmed all cases to be positive on pituitary-depended HAC. Five of the dogs were treated with titration dose of trilostane (trilostane-titration group-TTG), while two of the dogs were treated with the standard recommended dose (2.2-6.7 mg/kg once daily) (classic-treatment group-CTG) for up to two weeks after which the treatment was discontinued due to adverse effects. The titration dosing of trilostane was conducted initially with 0.5 mg/kg once daily for 7 days, and then with 0.5 mg/kg twice daily for 7 days. The trilostane morning dose was increased by 0.5 mg/kg daily for 7 days, following the same with the evening dose, achieving 4-6 mg/kg daily dose on the 14-th day and until the end of the treatment.
The dogs were monitored from the first day of the treatment until 12-weeks in a 4-week interval. The clinical symptoms were evaluated according to the owner’s anamnestic data and clinical findings on the monitoring days.
Laboratory analysesHematology analysis was performed on veterinary hematology analyzer Exigo (Boule, Sweden), using whole blood in EDTA tubes, sampled with venipuncture of
v. cephalica antebrachi externa. It included the following parameters with its respective abbreviations and reference ranges: erythrocyte blood count (RBC-5.50-8.50x10
12/L), packed cell volume (PCV–47-55%), hemoglobin (HGB–12-18 g/dL), platelets (PLT-200-500x10
9/L), white blood cells count (WBC-6-17x10
9/L), lymphocytes (LYM-1.2-5.0x10
9/L), monocytes (MON-0.3-1.5x10
9/L), neutrophils (NE-3.5 12.0x10
9/L), and eosinophils (EO-0.1-0.5x10
9/L).
Biochemistry parameters were analyzed from the serum samples, on automatic biochemistry spectrophotometer ChemWell 2910 (Awareness Technology INC, USA), according to the manufacturer’s instructions for each kit (Human, Germany). Biochemistry analysis included the following parameters with its respective reference ranges: alanine transaminase (ALT-8.2-57.3 U/L), aspartate aminotransferase (AST-8.9-48.5 U/L), alkaline phosphatase (ALP-10.6-100.7 U/L), glucose (GLU-3.0-6.6 mmol/L), total proteins (TP-54.1-75.2 g/L), albumins (ALB-25.8-44.7 g/L), globulins (GLB-21.6-37.0 g/L), cholesterol (CHL-3.1-6.5 mmol/L), triglycerides (TG-<1.14 mmol/L), creatinine (CRT-44.3-138.4 umol/L), urea (UREA-3.14-9.25 umol/L).
Statistical analysisThe values were expressed as mean ± standard error of mean. The within group comparison was performed by Friedman ANOVA. The between group comparison was performed by Mann Whitney U test. The significance level was set at p<0.05.
RESULTSClinical manifestationThe main clinical manifestations in all HAC patients (n=7) were polyuria and polydipsia. Dermatologic abnormalities (n=5), polyphagia (n=5), pendulous abdomen (n=4), muscle weakness (n=3), and panting (n=3) were also observed. The TTG dogs improved clinical signs following one month of the treatment, with complete resolution of clinical signs after 3 months. The two CTG dogs had neurological signs (discoordination, cranial nerve deficit with difficult swallowing, drooling and nistagmus). One of the CTG dog had hypersalivation, whereas the other had severe muscle weakness and continuous polyuria and polydipsia. In both dogs, the clinical signs exacerbated in the first two weeks after the treatment began.
Laboratory resultsThe PCV in the CTG patients was nonsignificantly lower and below the reference range at ~12 weeks (33.00%±2.60) by 19.00 and 17.85% compared to the 0- and ~4 week-treatment, respectively. The values in the TTG had no significant difference between the different periods of the treatment or the CTG (
Table 1).
The HGB value in the CTG was non-significantly lower and below the reference range at ~12 weeks (7.66%±6.34 g/dL) compared to the 0- and ~4-weeksamples by 9.69 and by 9.09 g/dL, respectively. The HGB values in the TTG were within the reference range and they were non-significantly different (p=0.0528) compared to the CTG or between the different stages of the treatment (
Table 1).
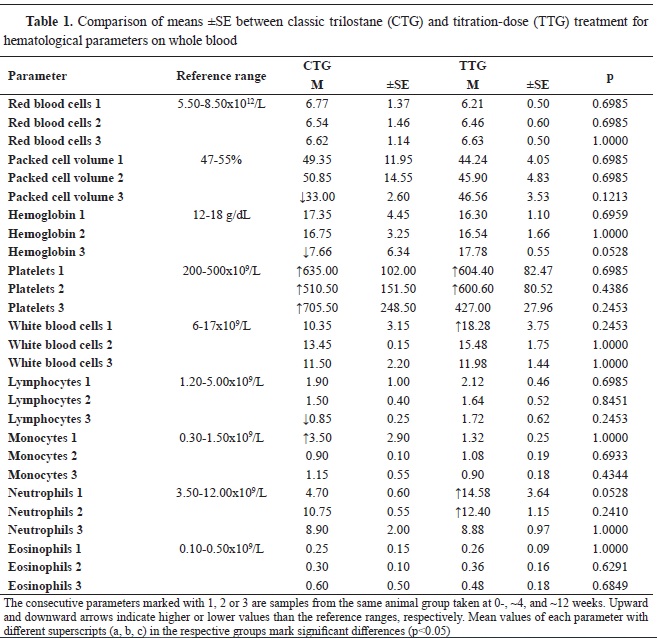
The NE in the TTG patients were above the reference range at 0- and ~4 weeks of the treatment and were non-significantly higher by 5.70 and by 3.52x109/L compared to the ~12 week-sample (8.88±0.97), respectively. However, it should be noted that the 0-week samples between the CTG (4.70±0.60) and TTG (14.58±3.64) group were highly but non-significantly different at p=0.0528 (
Table 1).
ALT had higher than the reference range values in both groups with significant decrease in the TTG group at ~12 weeks by 59.77 and 53.88 U/L compared to the samples at 0- and ~4 weeks, respectively.
Despite the markedly lower values at ~4 and ~12 weeks in the CTG by 73.63 and 79.82 U/L, respectively, these were non-significant compared to first sample at 0-weeks (226.87±122.30). No significant differences were observed between the CTG and TTG group in different periods during the treatment. Despite the markedly lower value in the TTG group at ~12 weeks (78.04±15.37) compared to the CTG (147.05±53.41), the difference was nonsignificant (p=0.2453) (
Table 2).
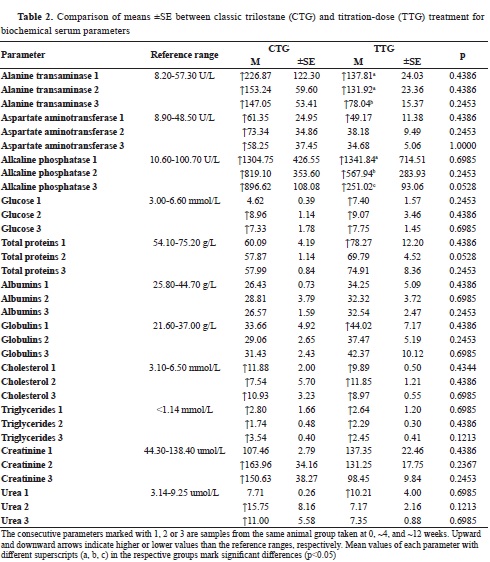
ALP had higher than the reference range in both groups with significant decrease in the TTG group at ~4 weeks by 773.90 and at ~12 weeks by 1,090.92 U/L compared to the first sampling at the beginning of the treatment (1,341.84). A non-significant decrease was observed in the CTG at ~4 and ~12 weeks of the treatment by 485.65 and 408.13 U/L, respectively compared to the initial value at 0-weeks (1,304.75). Despite the markedly lower value in the TTG group at ~12 weeks (251.02±93.06) compared to the CTG (896.62±108.08), the difference was non-significant (p=0.0528) (
Table 2).
The CRT level in the CTG was non-significantly higher and above the reference range at ~4 and ~12 weeks by 56.50 and 43.17 umol/L, respectively compared to the initial value at 0-weeks (107.46±2.79). The CRT level in the TTG group was within the reference range but had non-significantly lower value at ~12 weeks by 38.90 and 32.80 umol/L compared with the values at 0- (137.35±22.46) and ~4 weeks (131.25±17.75), respectively. There was no significant difference between the creatinine levels in any stage of the treatment between the groups (
Table 2).
The urea level in the CTG was above the reference range at ~4 and ~12 weeks of the treatment being non-significantly higher by 8.04 and by 3.29 umol/L compared to the initial sample at 0-weeks (7.71±0.26). In the TTG group, the urea level at ~4 and ~12 weeks was non-significantly lower by 3.04 and 2.86 umol/L compared to the 0-week sample (10.21±4.00). There was no significant difference between the urea levels in any stage of the treatment between the groups (
Table 2).
CHL and TG levels were elevated in both of the groups, but they were non-significantly different neither between them nor throughout the treatment (
Table 2).
DISCUSSIONThe current retrospective study included seven dogs with middle and terminal stages of HAC confirmed with LDDST. They had typical clinical symptoms of HAC with various comorbidities. Following reports for the adverse effect of the single-dose treatment with trilostane (
8, 10), the study aimed to investigate the hematological, biochemical, and clinical variations between dogs treated with the recommended trilostane dose and the titration trilostane dose.
HAC is a common endocrinopathy in dogs with variable clinical and laboratory findings. Depending on the clinical condition and duration of the disease, medical treatment with trilostane could give favorable effects in remission and resolution of clinical symptoms and can achieve good control of comorbidities in dogs with terminal stage of HAC. However, standard single-dose treatment may cause pituitary macro-adenoma which exacerbates the neurological signs as a result of ACTH retention in the endocrine producing cells. Mental dullness, salivation, lethargy, depression, anorexia, vomiting, diarrhea, weakness, discoordination, and distorted head with neck were the most commonly reported neurological symptoms in HAC-dogs treated with trilostane (
13).
Serum cortisol level has direct impact on the erythropoiesis affecting the RBC. Cortisol receptor in hematopoietic tissues have major role in the process of proliferation and differentiation of erythroid stem cells (
14). In the current study, the RBC did not show any significant difference between the CTG and TTG, but they were both in the reference ranges despite the various comorbidities. This could be explained by the fact that hypercortisolemia has favorable effect on the erythropoiesis in the proliferation and differentiation stages (
15).
Chronic exposure to elevated serum cortisol concentration can cause overloading and multisystemic impairments (
14).
HGB was higher in TTG at 12 weeks of the treatment samples, but due to the small sample size, did not present significant difference.
The elevated levels of PLT from the upper reference ranges in both groups, indicated secondary reactive thrombocytosis induced by high cortisol concentrations. This was likely due to the long-term proinflammatory reaction caused by the hypercortisolemia. Thrombocytosis is closely related with HAC resulting in increased tendency for clotting disorders. Cushing’s syndrome can also be associated with blood clotting disorders (
16).
The leucogram, especially the WBC, were not significantly different between the two groups. NE was above the reference range in the TTG at the beginning and at 4 weeks of the treatment, but has decreased to the reference range at 12 weeks. This could be explained with the neutrophilic effect of cortisol and also due to the persistent dermatology infection in these patients. High cortisol concentrations affect the white blood cells by causing lymphopenia, neutrophilia, monocytosis, and eosinopenia, which is known as “stress leucogram” that can be used as a reliable indicator for Cushing’s syndrome (
14) along with the clinical symptoms and anamnestic data. Hematology findings could not be considered specific in determining Cushing’s syndrome and can vary due to other comorbidities, but they may further assist in the easier diagnosis of the disease (
5).
Increased ALP, ALT activity, hyperlipidemia, and hyperglycemia are a common finding in dogs with Cushing’s syndrome (
14, 17, 18). In the current study, ALT, ALP, AST, and CHL were above reference ranges in both of the groups at the beginning of the treatment. The elevation of ALT, AST, and ALP levels above reference ranges is an indicator of increased gluconeogenesis and glycogenesis which is manifested as hepatic metabolic overload induced from the elevated cortisol concentration (
14). Elevated values of transaminases and ALP in the TTG might be due to poor adaptation of hepatic cells. However, ALT and ALP were significantly lower in the TTG at the end of the treatment compared to the 0-week samples. This could be explained due to the lowering effects of the trilostane on cortisol serum levels. However, there was no correlation between the treatment outcome and the serum ALP which is reflected with the higher-than-the-reference range of values (
19). In the study of Cho et all 2013, ALP, ALT, total CHOL and GLU concentrations were significantly lower after the initiation of low-dose trilostane treatment, but no significant differences were observed between the groups, except for the ALP activity (
18). In another study, significant decreased levels of ALP and ALT were observed at 1, 3, and 6 months after the initiation of trilostane treatment (
20).
Total proteins and albumins were not significantly affected in the dogs from both groups in the current study. Hyperlipidemia, presented by total CHOL and TG, exhibited consistently higherthan- reference values in both groups. This is clear indication of the long-term cortisol effect on lipid metabolic redistribution in the blood stream with persistent hyperlipidemia.
The renal status reflected by UREA and CREAT had non-significant increasing tendency in the CTG and non-significant decreasing tendency in the TTG group. It should be noted that renal impairment could be common comorbidity during the terminal stages of HAC in dogs and could be alleviated by trilostane titration-dose treatment.
Glycemic status should be considered with caution, especially in untreated HAC patients due to cortisol-induced hypothyroidism and insulindependent diabetes mellitus. Both groups in the current study had elevated GLU levels but without significant changes during the treatment or between the groups.
The current retrospective study has limited data due to the small sample size, but gives a clear indication for the success of the dose-titration treatment with trilostane in HAC dogs in the later stages of the disease.
CONCLUSIONThe single-dose trilostane treatment causes more adverse effects on dogs with hyperadrenocorticism and various comorbidities, rendering the patients unfit to withstand the whole treatment. The dose-titration treatment has indicated to have significantly higher tendency to decrease ALT and ALP during the later stages of the treatment, alleviating the negative effects on the liver. The clinical findings were more adverse in the single-dose treatment.
CONFLICT OF INTERESTSThe authors declare that they have no known conflict of interest in the conduction of the current study.
ACKNOWLEDGMENTSThe study was conducted with patients admitted at the University Veterinary Hospital–Faculty of Veterinary Medicine–Skopje, Ss. Cyril and Methodius University in Skopje with consent of the owners.
AUTHORS CONTRIBUTIONIC has conceptualized the study, has been treating the patients, collected the data, and contributed in the writing and formulating the final version of the manuscript. MN has designed the retrospective analysis of the data, performed the statistical analysis, and contributed in the results presentation of the manuscript. TN has treated the patients, monitored the clinical manifestations in the patients, collected samples, and contributed in the interpretation of the results in the discussion section. AA participated in clinical work for initial contact with owners and treatment protocol of the patients. IGj has collected and analyzed the samples, and contributed in the interpretation of the results in the discussion section. EAP has collected the samples, monitored and treated the patients, interpreted the hematological and biochemical findings, and supervised the manuscript writing and editing. All authors have approved the final submitting version of the manuscript.

 10.2478/macvetrev-2024-0016
10.2478/macvetrev-2024-0016

