Parasitic diseases of wild animals represent an important area of research. In addition to the significant impact on wildlife health and fitness, many parasitic diseases have zoonotic implications. Due to limited scientific information, this research aimed to investigate parasitic diseases in wildlife in Bosnia and Herzegovina (B&H), focusing on the Federation of Bosnia and Herzegovina (FB&H), emphasizing zoonotic species. In the period from April 2020 to November 2022, we conducted research on 9 wildlife species. We analyzed fecal samples to detect and identify diagnostic stages (eggs, larvae, cysts, and oocysts) of various animal endoparasites using coprological methods, such as sedimentation, flotation, and the Baermann technique. The MERIFLUOR® Cryptosporidium/Giardia test was also used for the detection of Cryptosporidium oocysts and Giardia cysts. In the case of red foxes, intestinal samples were examined using the intestinal scraping technique to detect adult helminths. All collected muscle samples were subjected to the artificial digestion method for Trichinella detection. From 1,278 samples, 70.9% were positive. Parasitic infections were confirmed in 15.9% (11/69) of bears; 83.7% (262/313) of red foxes; 67.6% (44/65) of wolves; 25% (1/4) of wildcats; 20% (1/5) of badger; 43.7% (7/16) of martens; 39.7% (76/191) of wild boars; 84.5% (350/414) of deer, and 77.1% (155/201) of hares. The finding of zoonotic parasites (Toxocara canis, Uncinaria spp., Trichinella spp., Echinococcus spp. etc.) is particularly important due to their potential detrimental effects on human health, which highlights the need for further investigations.
The proportion of forest lands in the Federation of Bosnia and Herzegovina (FB&H) is larger than the agricultural lands, and they are the ideal habitat for different wildlife species (
3). In addition, the ongoing urbanization process and converting natural habitats into agricultural land are factors that reduce or move imagined barriers between wildlife, domestic animals, and people facilitating the exposure of new naïve hosts to different types of parasites. For example, red foxes (
Vulpes vulpes) play an important role in the transmission of parasites to domestic animals and are natural hosts for a large number of zoonotic parasitic species (
4). Similarly, interactions between wildlife and domestic animals, as well as the transmission of parasitic infections, are quite probable in regions with significant populations of free-roaming dogs, cats, and livestock. Studies investigating endoparasites among wild animals have been extensively carried out across various European countries, including Greece (
1), Switzerland (
5), Slovenia (
6), Spain (
7), Germany (
8), Poland (
9), etc. These research endeavors underscore the paramount significance of zoonotic species and their pivotal role in the transmission of parasites to domestic animal populations.
Bosnia and Herzegovina (B&H) has a diverse wildlife. However, there are no official or estimated data on abundance or type of wildlife. Likewise, research studies of wildlife parasitic infections in the FB&H are rare and mainly related to the presentations of individual or flock/herd cases in the limited areas of the Federation. These areas are primarily designated as protected areas, including nature monuments, national parks, and nature reserves.
Wild animals move freely through various territories of FB&H, thus increasing the possibility to transmit parasitic infections beyond their wellestablished habitat. This fact implies that monitoring wildlife health is crucial for two reasons: ensuring the survival and conservation of wildlife species by reducing the burden of parasitic diseases as well as decreasing public health risks of zoonotic species of parasites.
Given the impact that parasitic infections can have on the health of wild animals, domestic animals, and humans, and considering that no information is available regarding the parasitic fauna of FB&H, the aim of the present study was to investigate the parasites of wildlife in the FB&H with an emphasis on zoonotic species.
MATERIAL AND METHODSStudy areaBosnia and Herzegovina (B&H) is located in the western part of the Balkan Peninsula with the total area of 51,209 km
2. The country is administratively composed of the Federation of Bosnia and Herzegovina (FB&H), Republika Srpska (RS), and the Brčko District. B&H is a very mountainous country in the frame of the mountain system of Dinarides (Dinaric Alps). Almost the entire FB&H (26,110 km²) is located in the Dinarides. It is mainly forested except for the plains of the Posavina region of the Pannonian Plain. Forests and forest land cover 53% of the territory of B&H, of which forests cover about 43% and barren land with degraded forest about 10%.
Sample collection and investigationsIn the period from April 2020 to November 2022, a total of 1,278 samples were analyzed, including feces, muscle tissue, and intestinal samples collected from 41 sites within FB&H registered hunting grounds (
Fig. 1). Samples were collected from a total of 9 wildlife species: bear (
Ursus arctos, n=69), fox (
Vulpes vulpes, n=313), wolf (
Canis lupus, n=65), wildcat (
Felis silvestris silvestris, n=4), badger (
Meles meles, n=5), marten (
Martes martes, n=16), wild boar (
Sus scrofa, n=191), roe deer (
Capreolus capreolus, n=414), and brown hare (
Lepus europaeus, n=201). The members of the hunting organizations in cooperation with veterinary organizations in the FB&H were collected and continuously delivered samples in the Laboratory for Parasitology (accredited within BAS/EN ISO/IEC 17025:2018) of Veterinary Faculty, University of Sarajevo, where standard parasitology techniques were performed. In addition, samples were also collected from dead or killed wild animals during necropsy.
A total of 1,129 fecal samples were collected in the field. Each fresh fecal sample was placed into a sterile vial (a 50 mL). The minimal weight of each sample was 20 g. The identification of animal species was based on specific morphological features observed in the collected feces, such as color, shape, size, and volume, as well as characteristic defecation habits, including location, frequency, and animal tracks.
Intestinal samples (n=57) were collected during the necropsy of red foxes that were shot during the national program for evaluation of the effectiveness of the anti-rabies vaccine. Intestinal samples were frozen at −80 °C for at least seven days before processing. This biosafety procedure was performed to prevent the possible transmission of zoonotic pathogens to the investigators (
10). Afterwards, the samples were thawed and utilized for subsequent parasitological investigations.
Muscle samples (diaphragm and masseters) of wild boars (n=88) were collected during the hunting seasons in the period 2020-2022, while the muscle samples (diaphragm) of bears (n=3) and badgers (n=1) were collected during the necropsy. The minimal weight of each collected muscle sample was 100 g.
Fecal and intestinal samples were examined macroscopically to determine the presence of adult parasites. Additionally, fecal samples were tested using coprological methods to detect and identify parasitic stages (eggs, larvae, cysts, and oocysts), e.g.: sedimentation (
11, 12), flotation (
11, 13), Baermann technique (
14), and direct immunofluorescence test (MERIFLUOR®
Cryptosporidium/Giardia test (Meridian Bioscience Inc.) for detection of
Cryptosporidium oocysts and
Giardia cysts according to the manufacturer’s instructions (
15). The Baermann technique was used to detect the first larval stages (L1) of lungworms. The intestinal samples from red foxes were analyzed using the intestinal scraping technique (
16) to detect adult helminths (cestodes and intestinal nematodes). All collected muscle samples were analyzed for the detection of
Trichinella spp. using the artificial digestion method as per Commission Regulation (EC) No. 1375/2015 (
17) and OIE guidelines (
18).
Determination of the parasite species was based on the microscopical assessment of morphological characteristics of parasitic forms (i.e., eggs, larvae, oocysts, cysts) observed in the samples using two types of microscopes: Olympus CH20 BIMF200®, and fluorescence microscope Olympus BH-2-RFCA® (Magnification 100×, 400×, and 1,000×) alongside a comparison with given parameters specified in the diagnostic method manuals (
8, 13, 19, 20).
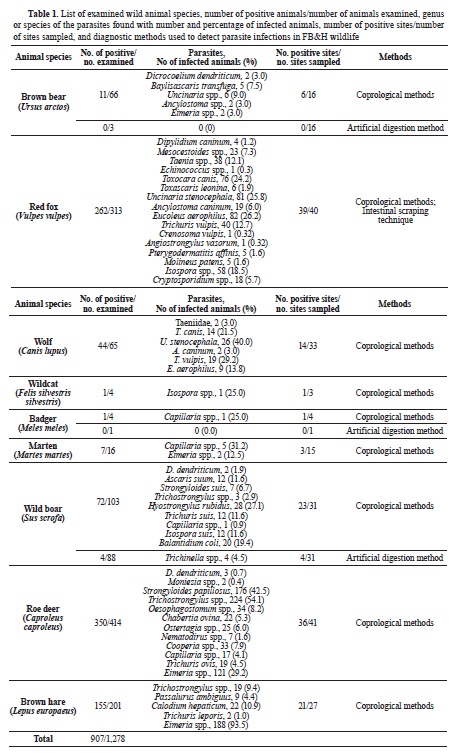
RESULTSOf the 1,278 samples collected from 9 species of wild animals belonging to three orders (Carnivora, Artiodactyla, Lagomorpha), a considerable percentage, equal to 70.9% (907/1,278), demonstrated the presence of various parasitic elements (eggs, larvae, cysts, and oocysts). Parasitic infections were confirmed in different proportions among the examined species: 15.9% (11/69) of the bear population, 83.7% (262/313) of red foxes, 67.6% (44/65) of wolves, 25% (1/4) of wildcats, 20% (1/5) of badgers, 43.7% (7/16) of martens, 39.7% (76/191) of wild boars, 84.5% (350/414) of deer, and 77.1% (155/201) of hares. The identification of parasites was performed to family, genus or species level. A detailed overview of the results is given in
Table 1. A mixed infection with multiple parasite species was identified in the majority of examined animals.
 DISCUSSION
DISCUSSIONScientific information and basic knowledge of the parasitofauna of wildlife are limited, both in B&H and in many other countries. However, the intensification of research in the last decade highlights the importance of certain parasitic species for veterinary and public health. Moreover, research activities influence a better understanding of the effects of parasites on wildlife, domestic animals and humans (
17, 20, 21). In this regard, a more comprehensive examination of the parasitofauna in wild animals in the FB&H is needed, and research should be specifically directed to new areas, biotopes, and animal species.
To the best of the authors’ knowledge, this study represents the first comprehensive research conducted on several wildlife species across various locations in the entire B&H. This research identified parasitic species that could have a detrimental role in the preservation and well-being of wildlife populations. Notably, parasites affecting specific wildlife species, such as canids, felids, and ruminants, hold greater relevance concerning the health dynamics of domestic animals due to their taxonomic interconnection, thereby facilitating the presence of shared parasitic taxa. Furthermore, the identified parasitic species could be easily transmitted and introduced into the population of domestic animals and humans. Similarly, Karamon et al. (
9) claimed that most parasitic species found in mesocarnivores (red foxes, wolves, and wildcats) could be transmitted to domestic animals, i.e. dogs, cats, and vice versa. Nevertheless, the most important fact of these findings was that most parasitic species also had zoonotic significance (
9).
Besides the anthropogenic factor that significantly affects the natural habitat and sustainability of the European brown bear population in a particular area, parasitic infections can drastically affect the general health, fitness, and reproductive abilities of bears (
23). In addition to having an adverse effect on the individual animal or population, many parasites also have zoonotic potential. In this regard, as part of the research, special attention was paid to determining the health status of brown bears in the FB&H, considering the limited information from previous studies on gastrointestinal parasites in bears (
23). Many European studies have reported a high percentage of parasitic infections of free-living and brown bears kept in captivity with different parasitic species such as:
Uncinaria spp.,
Dicrocoelium spp.
Trichuris spp., and
Giardia spp., while the special focus was on
B.
transfuga (
24, 25).
The presence of
B. transfuga was also confirmed by our research in 7.5% of the tested bear samples. The presence of
D. dendriticum,
Ancylostoma spp., and
Eimeria spp. was confirmed in 3.0% of investigated bears, while the most represented parasitic species among the brown bear population was
Uncinaria spp., present in 9.0% of the samples. In the territory of neighboring Croatia, the presence of
B. transfuga, Ancylostoma spp.,
Uncinaria spp.,
Taenia spp.,
Giardia spp.,
Cryptosporidium spp., and
Eimeria spp. was confirmed in 33% of 94 samples originated from free-living brown bears (
26). Furthermore, the presence of the potentially zoonotic intestinal nematode
B. transfuga was confirmed by parasitological and molecular methods in brown bears in Slovenia and Slovakia with an incidence rate of 52.9% (
27). In the same study, Štrkolcova et al. (
27) have reported other parasitic infections in brown bears including:
Ancylostoma spp.,
Toxascaris spp.,
Cryptosporidium spp.,
Taenia spp., and
Capillaria spp.
However, most scientific data on the presence of
B. transfuga in free-living bear populations come from the American, Canadian, and Russian populations of black, brown, and polar bears (
24, 28). The data on the presence of
B. transfuga in populations of brown bears in Europe are still relatively scarce (
26, 29, 30).
The results of this study revealed a worrying number of red foxes infected with various zoonotic parasitic species in FB&H and the results of our research indicate that red foxes and wolves are significant reservoirs for parasitic species including
Echinococcus spp.,
T. canis, T. leonina,
U. stenocephala, A. caninum, T. vulpis, E. aerophilus,
P. affinis,
M. patens, and
Cryptosporidium spp. The presence of
T. canis in red fox populations has been recorded throughout Europe with percentages varying between 26.7 and 66% (
31). In our study,
T. canis was detected in 24.2% of the tested red foxes, and in 21.5% of the tested wolves. The relatively high prevalence of
T. canis detected in the populations of red foxes and wolves in FB&H indicates the possibility of transmission of the parasite to other wildlife species, domestic animals, or humans. In addition, we detected the presence of
P. affinis and
M. patens for the first time in population of red foxes in FB&H (1.6% of the tested red foxes).
In our study, the presence of
U. stenocephala was more frequent than the presence of
A. caninum and other endoparasites in the examined populations of red foxes and wolves. In the red foxes and wolves included in the study,
U. stenocephala was detected in 25.8% and 40% of the samples, respectively.
A. caninum was detected in 6% and 3% of the red foxes and wolves examined, respectively. Similarly, the presence of
U. stenocephala in red foxes across Europe was 34% (
32), although a significantly lower prevalence of this parasite (14.8%) was detected in Serbia (
33). Furthermore,
U. stenocephala was also detected in 41.2% of wolves examined in Latvia (
34). These nematodes have the potential to induce enteritis, skin lesions, and cutaneous larva migrans syndrome in humans, posing a threat to human health.
E. aerophilus is considered one of the most common lungworms in wild carnivores in Europe. In this research,
E. aerophilus was detected in 26.2% of samples originating from red foxes, and 13.8% of samples originating from wolves, while
A. vasorum and
C. vulpis were detected in 0.3% of samples originating from red foxes only. Our results are in accordance with the results of a previous study on
E. aerophilus that showed a relatively high prevalence of this parasite in European carnivores, ranging between 9 and 36% (
35). Other studies have shown a diverse prevalence of
E. aerophilus in red foxes in the Pyrenees and Serbia of 30% and 84%, respectively (
36, 37). Furthermore,
E. aerophilus infections were detected in 36.4% of wolves in Latvia. Its presence in wildlife is notable not only due to their potential impact on cats and dogs but also because they pose a potential zoonotic risk, occasionally leading to severe implications for human health (causing human capillariosis).
In this study, the overall prevalence of
Cryptosporidium spp. in the population of red foxes was 5.7%, which is similar to the results of researches conducted in Canada, Croatia, Iran, Ireland, Norway, Spain, the UK, and the USA. The prevalence found in these studies ranged between 0.4%-16.0% (
7, 38).
As observed in our study, wild boars are infected with parasites that not only represent a potential health problem for domestic pigs (
Trichostrongylus spp.,
S. suis, T. suis, H. rubidus, Metastrongylus spp.,
B. coli) but also represent a potential public health risk (
B. coli, A. suum, T. suis, Capillaria spp.,
Trichinella spp.). The most frequent parasitic infections in wild boars in FB&H were
H. rubidus (27.1%) and
B. coli (19.4%). A particular concern is the relatively high prevalence of zoonotic B. coli found in the tested samples from wild boar, which, together with domestic pigs, are considered reservoirs of infection. The species
A. suum and
T. suis were detected in 11.6% of the samples. Higher prevalence were previously recorded in wild boars in Poland.
T. suis was found in 13.4% of wild boars and
A. suum in 15.5% (
39). Furthermore, this study included the examination of wild boars for
Trichinella spp. From 88 muscle samples (diaphragm and/or masseter), 4 (4.5%) were positive on
Trichinella spp. It’s important to emphasize that there is no systematic surveillance for
Trichinella spp. in FB&H wildlife, even though the results of some previous studies show a high prevalence of this parasite in various wildlife species (
40).
In our research, the presence of parasites was detected in 84.5% of samples originating from roe deer, which is several times higher than results reported from studies in Switzerland (12%) (
5) and Slovenia (48%) (
6). The relatively high prevalence of parasitic diseases in the population of roe deer in FB&H represents a severe problem for the preservation and well-being of these wildlife species. This epidemiologic situation can be linked to the nomadic and semi-nomadic rearing of small ruminants, which is still present in B&H. The direct contact between domestic and wild ruminants, the indirect contact by sharing the same pastures, and the absence of systematic monitoring of parasitic diseases in domestic and wild ruminants, can be considered determining factors for the high prevalence of parasitic diseases in the population of wild ruminants in FB&H.
All parasitic species of roe deer reported in the current study were also reported in studies conducted in Switzerland and Slovenia (
5, 6). In FB&H roe deer, the most prevalent parasites were
Trichostrongylus spp. (54.1%),
S. papillosus (42.5%), and
Eimeria spp. (29.2%).
Reports on the presence of endoparasites in populations of European hare in Europe are sporadic. Parasitic infections were detected in 77.1% of samples originating from brown hares in FB&H. Our results showed the presence of
Trichostrongylus spp.,
P. ambiguous, C. hepaticum, T. leporis, and
Eimeria spp. Our findings call for special attention on the parasitic species
C. hepaticum which can be transmitted to susceptible domestic animals and humans via contaminated vegetation.
CONCLUSIONOur research was geared towards the detection and identification of parasitic species in wildlife in FB&H, and our results indicate that such parasitic fauna includes a wide variety of species. Our findings also indicate the potential risk of transmission of detected parasite species from wildlife to domestic animals and humans. Therefore, the zoonotic potential and other veterinary and public health aspects of the identified parasite species should not be overlooked. Given that the examined animals are widely distributed in the FB&H territory, the migration of wild animals to neighboring countries, urbanization, and the adaptation of wild animals to urban areas increase the risk of parasite transmission to domestic animals and humans. In this regard, it is necessary to carry out further research, and there is need for continuous monitoring of parasitic diseases of wildlife, with a particular emphasis on zoonotic species.
AUTHORS’ DISCLOSUREThe authors disclose that this manuscript has been uploaded as a preprint version (non-peer-reviewed work) before submission to Macedonian Veterinary Review. The preprint can be found at:
https://doi.org/10.21203/rs.3.rs-2669579/v1 CONFLICT OF INTERESTThe authors declare that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGMENTSThe authors would like to express their sincere gratitude to all who contributed to the realization of this study. This study was supported by the Veterinary Faculty and the Faculty of Agriculture and Food Science, University of Sarajevo.
AUTHORS’ CONTRIBUTIONAll authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JO, NK, VŠ, AS, ŠG, EŠ and TG. The first draft of the manuscript was written by NK and all authors commented on previous versions of the manuscript. All authors reviewed the manuscript, furthermore, all authors read and approved the final manuscript.

 10.2478/macvetrev-2024-0017
10.2478/macvetrev-2024-0017

