Combining analgesic medications with distinct pharmacodynamics may result in effective analgesia and reduced adverse effects by lowering dosages of one or both medications. The objective was to investigate the nature of interactions of metoclopramide and metamizole by measuring the antinociceptive and neurobehavioral effects in mice. The drugs were administered intraperitoneally (ip) in mice. The antinociceptive effect was determined by the up-and-down method. Isobolographic analysis was performed by simultaneous injection of the two drugs at ED50 ratios of 1:1 and 0.5:0.5. The treated mice were also subjected to an open-field behavioral test as well as dorsal immobility and negative geotaxis behavioral paradigms. The ED50 of metoclopramide and metamizole were 33.71 and 43 mg/kg ip, respectively. Lower ED50 was established for metoclopramide and metamizole by 48.44% and 47.49%, respectively when given at a ratio of 0.5:0.5, and by 35.81% and 29.88%, respectively at a ratio of 1:1. The combined dose of metoclopramide and metamizole (8 and 10 mg/kg, ip, respectively) significantly decreased the open field-activity test in mice. This was observed by lower number of squares crossing compared to the control. Additionally, the drug combination resulted in a substantial increase in the duration of dorsal immobility response and delayed the negative geotaxis task when compared with the control group. The results indicated that the interaction between metoclopramide and metamizole in lower dose ratio with ED50 50:50, was synergistic in producing analgesia in mice with reduced risk for adverse reaction but with decreased behavioral and motor neuronal activity.
Dopamine D2-receptor antagonist (metoclopramide) has been utilized as an antiemetic and gastroprokinetic drug acting in the central nervous system CNS (chemoreceptor trigger zone) in humans (
1) and animals (
2). The medication has also indirect cholinergic action (blocks the release of acetylcholine from intrinsic cholinergic neurons) (
3) and an agonist serotonergic 5-HT4 receptor effect (
4). Metoclopramide has been shown to have a centrally produced depressive effect known as sedation reported in both human (
5) and hens (
6). Several investigations have documented the drug’s analgesic effects in humans, rats (
7), dogs (
8), and mice (
9), even though metoclopramide’s potential analgesic impact is still up for question. The medication has a good analgesic effect when combined with other analgesics such as morphine (
6), ketamine, diphenhydramine (
10), and xylazine (
11). Metamizole acts centrally (
12), however, the way it produces analgesia is not completely understandable yet. Analgesic influence of metamizole relay on its inhibitory effect of type 3 COX of the cyclooxygenase enzyme. That restrict the synthesis of prostaglandin in the spinal cord’s dorsal horn (
13, 14).
It also interacts with the glutamatergic system and release of endogenous opioid peptides (
15). Additionally, it has been predicted that CB1 cannabinoid receptors in the CNS are the means through which metamizole and its metabolites produce their analgesic effects (
16). Analgesics are used for treating acute pain in clinical and research settings. Paracetamol, ketoprofen, morphine, and xylazine are analgesics with different mode of action compared to metamizole (
17) and medetomidine. They have lower side effects and can have a synergistic antinociceptive impact when combined (
18).
This study aimed to assess the antinociceptive and neurobehavioral effects of metoclopramide and metamizole combination.
MATERIAL AND METHODSEthical statementThe Committee of Postgraduate Studies at the College of Veterinary Medicine, University of Mosul, Iraq, officially approved the study protocol in accordance with the institutional policies on animal handling and usage under the following code: UM.VET.2022.034, on Jan 25, 2023.
AnimalsForty-five adult Swiss mice of either sex, weighing 20-30 g, were used for this study. All mice were brought from Mosul University (College of Veterinary Medicine). They were housed in cages of the animal house under standard conditions, at regular temperature of 22-24 °C and 10/14 hour-light/dark cycle. The animals were given free access to tap water and routine laboratory meals. They were acclimatized to laboratory conditions for one week prior to the start of the experiment. The study protocol was approved by the Scientific Committee of the Department of Pharmacology and Toxicology at the College of Pharmacy, University of Mosul. All the animals were handled in accordance with the internationally accepted standard guidelines for use of laboratory animals (
19).
Drugs and dose preparationMetoclopramide (pure powder, Vainkunth Co., India) and metamizole (Saudi Pharmaceutical Industries, Riyadh, KSA 50% solution for injection), were freshly diluted in normal saline solution. They were administered intraperitoneally in a 5 ml/kg dose with a needle (gauge 31).
Determination of median effective dose (ED50) for metoclopramide and metamizoleThe median analgesic effective dose (ED50) of metoclopramide and metamizole was determined by the up-and-down method (
19) (separately or jointly) by the employing hot plate (thermally induced pain method) in mice. The first dose of metoclopramide and metamizole was obtained depending on our pilot experiment.
A 25% of the initial dose for both drugs was used as a starting point for increasing or decreasing the doses. The latency was recorded immediately after gently putting the mice on a heated surface of a hot plate (Heidolph Me, Germany, Hei-standard Co.) at 55-56 ºC. The latency is the time between placing and withdrawing the mice from the hot plate surface. The withdrawal may include jumping off or paws licking indicating pain response. The reaction time and response were recorded by using a stopwatch (Bright Medi Weld Appliances, China).
The mice that failed to react to heat nociceptive response within 30 sec (cut off times) were excluded from the experiment to minimize or to avoid tissue damage (
20). Each mouse was used once. The pain threshold for each mouse was assessed at the start of the test (base line) and at 15 min after each treatment. Following the heat plate exposure, the paws were placed in water at room temperature to relieve the pain experienced after the experiment.
Identification of metamizole and metoclopramide’s analgesic interactionsThe metoclopramide and metamizole interactions on the antinociceptive level was assessed in seven randomly chosen mice. One mouse was initially treated intraperitoneally in 1:1 ratio of the ED50, in doses of 34 mg/kg and 43 mg/kg, and then in 8 and 11 mg/kg, respectively. The other five mice were treated intraperitoneally in 0.5:0.5 of ED50, initially in doses of 16 and 20 mg/kg, and then in 4 and 5 mg/kg, respectively. These treatments were conducted during the hot plate test.
Isobolographic analysis and Y interaction index were used to detect the relationship between the two medications in their respective dose ratios (
21, 22) by crossing the ED50 value of metoclopramide (x-axis) and metamizole (y-axis). The yielded value represented the ED50 for the combination of two drugs at 1:1 and 0.5:0.5 dose ratio. ED50 values below and above the diagonal line represented synergistic and additive (no interaction)/antagonist relationship, respectively.
The interaction index (Y value) was estimated with the following equation: Y=da/Da+db/Db, where: Da and Db are the individual ED50 of metoclopramide and metamizole respectively, da and db are the mixture of dosages that have the same analgesic effect. The interpretation of the interaction index was as follows: Y=1, additive; Y<1 synergistic (supra-additive); Y>1 antagonistic (sub additive) interaction.
Effect of single or combined treatment with metoclopramide and metamizole on neurobehavioral and locomotor activity
The animals (n=20) were divided into four groups of 5 mice. Each mouse was injected intraperitoneally with normal saline (control) or with metamizole (10 mg/kg) and metoclopramide (8 mg/kg). The doses were established according to the previous assessments for ED50 and Y so they would not induce overt effects of sedation or analgesia. Following 15 min of the treatment, each mouse was gently placed on the center of open field arena (25x35x35 cm, divided into 15 squares) to measure the 3-min open-field activity as described before (
23).
The frequency of urination and defecation was recorded (
24). Animal behavioral changes were also recorded: cleaning, grooming, sniffing, licking, biting, head bobbing, and grouching (
25). The occurrence frequency of each behavior was scored as follows: 1-2=1 times, 3-4=2 times, 5=>3 times.
Each mouse was subjected to negative geotaxis test immediately after the open field activity test, which assessed the neuromotor coordination and spatial orientation (
26). The mouse was placed on a surface at 45° angle with lowered head. The time for complete 180° rotation to the normal position was recorded.
Dorsal immobility response test was conducted immediately after the negative geotaxis test to assess the analgesic effects (
27). The mouse was gently raised by folding the skin between the ears and behind the neck. The time of resisting or escaping behavior occurrence was recorded.
Statistical analysisMicrosoft Excel was utilized for organizing and coding the data. SPSS version 21 (IBM) was utilized for data analysis (one-way analysis of variance) followed by the least significant difference test (
28). Non-parametric data (scores) were subjected to Mann-Witney U test. The data were considered statistically significant at p values <0.05. All the data expressed as mean ± standard error.
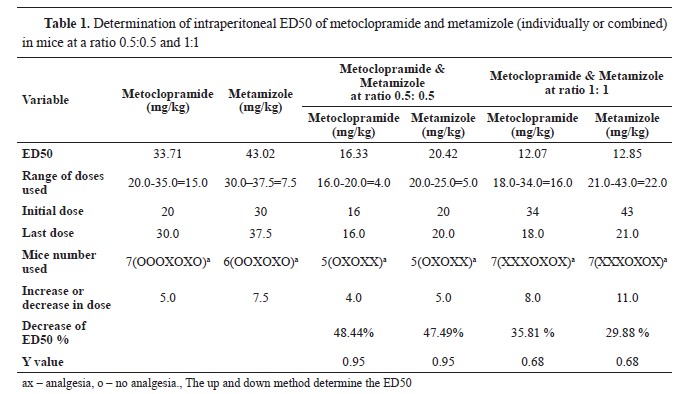
RESULTSDetermination the median effective dose (ED50) for metoclopramide and metamizole The ED50 of metoclopramide and metamizole which produced analgesic effect in mice was 33.71 and 43 mg/kg intraperitoneally, respectively (
Table 1).
 Identification of metamizole and metoclopramide’s analgesic interactions
Identification of metamizole and metoclopramide’s analgesic interactionsThe ED50 values for metoclopramide and metamizole at a ratio of 0.5:0.5, decreased 48.4% and 47.4%, respectively, and at 1:1 ratio, 35.8% and 29.8%, respectively (
Table 1).
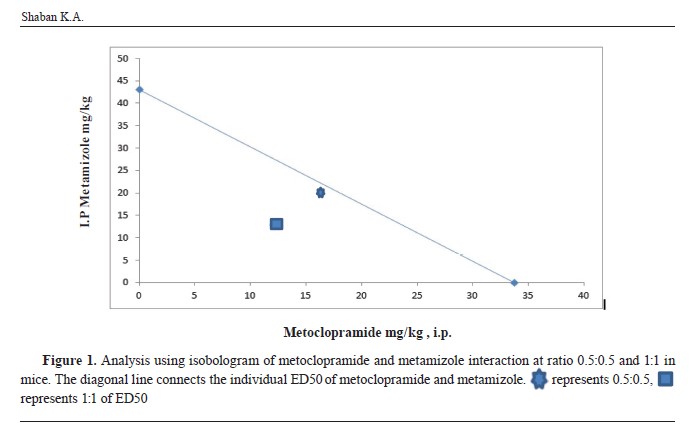
The value of ED50 for both ratios was located below the diagonal line between the individual ED50 of metoclopramide and metamizole. This indicated a synergistic antinociceptive interaction between the two drugs (
Fig. 1). The value of Y which was obtained from the interaction index equation, was lower than 1 (0.95 and 0.68) for the 0.5:0.5 and 1:1 ratio, respectively. This value was adopted as supportive indicator of synergistic interaction between two drugs.
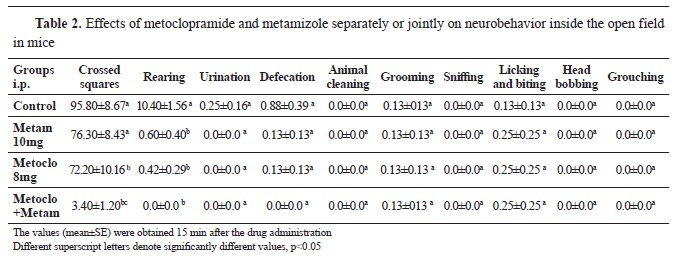
Evaluation the neurobehavioral effect and locomotor activity for metoclopramide and metamizole each alone or as combinationIntraperitoneal injection of metoclopramide (8 mg/kg), metamizole (10 mg/kg), and a combination of both, significantly decreased the number of rearing in the open field box compared to the control (
Table 2). Metoclopramide (8 mg/kg) significantly decreased the squares crossed by mice compared to the control group, within the period of 3 minutes (
Table 2). Combined intraperitoneal administration of metoclopramide (8 mg/kg) and metamizole (10 mg/kg) significantly decreased the number of squares crossed compared to the control, metoclopramide (8 mg/kg), and metamizole (10 mg/kg), respectively. There was no significant difference in the stereotype behavior among the groups (
Table 2).
 Neurobehavioral tests
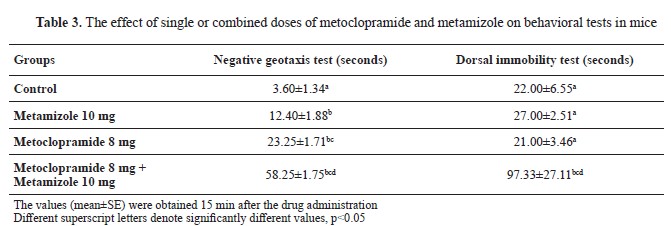
Neurobehavioral testsMetoclopramide 8 mg/kg applied intraperitoneally, significantly delayed the performance of negative geotaxis in comparison with control and metamizole 10 mg/kg values (
Table 3). Combined intraperitoneal injection of metoclopramide 8 m/kg and metamizole 10 m/kg significantly delayed the performance of negative geotaxis and significantly increased the time of dorsal immobility response in comparison with control, metoclopramide 8 mg/kg, and metamizole 10 mg/kg groups, respectively. Metamizole 10 mg/kg applied intraperitoneally, significantly delayed the performance of negative geotaxis compared with the control group (
Table 3).
DISCUSSIONThe current study initially determined the median ED50 for intraperitoneal administration of metoclopramide and metamizole in mice by applying the up-and-down method, which was 33.71 and 43 mg/kg, respectively. This dose is consistent with previous reports in the same animal model for metamizole (
16) and metoclopramide. Isobolographic analysis (
9, 10) and several combination ratios (1:1 and 0.5: 0.5 of ED50 for each drug) were used to assess the pharmacological relationship between metoclopramide and metamizole and its antinociceptive effect. This is the first report to quantify the antinociceptive impact of metamizole and metoclopramide co-administration. Synergistic interaction was identified between the two medications and was validated by calculating the Y interaction value, which was <1 (
20). The proposed mechanism of synergistic interaction between the two medications may be related to their differing mechanisms of action on pharmacodynamic level.
Metoclopramide acts as an antagonist on the D2 receptors which are connected to the opioid system. It contributes to the antinociceptive effect without interfering with the opioid receptors. It also elevates prolactin levels which are connected to the opioid system. Metoclopramide acts as a serotonin agonist (5-HT4 receptor agonist) (
29, 30) and it has another analgesic mode of action that is correlated with the calcium membrane transport (
31). Metamizole produces antinociceptive effect by initiating the neuronal nitric oxide. Nitric oxide release activates the L-arginine/nitric oxide/ cGMP/K+ channel pathway (
32). Activation of the descending inhibitory pain control system and the interaction with the glutamatergic system, are another mode of analgesic effect of this medication.
These synergistic analgesic interactions are in agreement with previous studies on metamizole with D-propoxyphene (a commonly prescribed opiate analgesic) (
33) and with morphine (
15). This indicates lower occurrence of metamizole side effects. Agranulocytosis and blood dyscrasia are frequently reported side effects of this medication (
34).
The current study supported the synergistic interaction between these two drugs by applying neurobehavioral and locomotor tests. The locomotor activity test in the open field arena is frequently used to measure general activities for laboratory animals (e.g., mice and rats). It is commonly used for observing and recording subtle effects in low-dose drugs acting on the central nervous system (e.g., metamizole and metoclopramide) (
35).
The current study assessed the antinociceptive effect of intraperitoneal administration of metoclopramide (8 mg/kg) and metamizole (10 mg/kg) in mice. These were sub-analgesic doses which had no visible impact on animal behavior. The inhibitory effect of the two drugs was observable by changes in the neurobehavioral and locomotor activity during the open field arena test in which the mice crossed fewer squares and had lower rearing. Additionally, they needed a longer time for negative geotaxis test performance and they had significantly longer dorsal immobility response test. These findings confirmed the inhibitory effect on the level of central nervous system.
The combined administration of both medications in the same dose yielded even higher inhibition response in these tests. This was similar to the findings of an earlier study with xylazine and metamizole combination in mice and in chicks (
9, 10). It should be noted, that these effects of the combined drugs were not significantly different since the doses were below the estimated ED50. The synergistic inhibitory effect on the neurobehavioral and locomotor activity may be attributed to the metamizole inhibitory effect on CNS (
36) and indirect activation of the central opioid receptors. Metoclopramide produced their inhibitory effect by increasing the prolactin level related to the opioid system, and by their antidopaminergic action in CNS (
37).
CONCLUSIONMetamizole and metoclopramide interacted synergistically in their antinociceptive effect in mice observed by decreased neurobehavioral and locomotor activity. The analgesic effect was achieved with lower individual doses, thus reducing the risk of adverse effects.
CONFLICT OF INTERESTThe authors declare that they have no known conflict of interest in the conduction of the current study.
ACKNOWLEDGMENTSThe University of Mosul, the College of Pharmacy, and the College of Veterinary Medicine are gratefully acknowledged by our study team for their aid, direction, and support.
AUTHORS’ CONTRIBUTIONZSH and KAS conceived, planned and carried out the experiments. ZSH took the lead in writing the introduction of manuscript. KAS took the lead in writing the methods and results of manuscript and analysed the data. GAF took the lead in writing the discussion of manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.

 10.2478/macvetrev-2024-0027
10.2478/macvetrev-2024-0027



