This work aimed to evaluate the lactoferrin (LF) effect on arsenic (As) and imidacloprid (IMI) toxicity in broiler chicks. One-week old broiler chicks (n=105) were divided into seven groups (x15 each). The animals were orally supplemented with As, IMI, and/or LF for 4 weeks as follows: Control (G1) no supplements, G2 supplemented with As, G3 supplemented with IMI, G4 supplemented with As+IMI, G5 supplemented with As+LF, G6 supplemented with IMI+LF, G7 supplemented with As+IMI+LF. Body weight and weight gain were recorded on weekly interval. Blood, serum, liver, kidney, and muscle samples were collected at the end of the experimental period for biochemical and histopathological examination. Body weight performance, hematological, serum, and liver tissue biochemical analysis revealed adverse changes in G2, G3, and G4 compared to control, G5, G6, and G7. There was higher tissue residue of As and IMI in G4 compared to G5, G6, and G7. Liver histopathological changes in the groups supplemented with As and/or IMI were observed with necrosis, congestion, and inflammatory cell aggregates. The use of LF in broiler chicks improves weight gain performance and modulates the adverse effects of As and/or IMI toxicity.
Arsenic (As) is one of the heavy metals that could be classified as a widespread trace element. Besides its beneficial impacts, it is also known to be very hazardous. Arsenic may exist in two prevalent and toxic inorganic forms, arsenate (pentavalent) and arsenite (trivalent). Arsenite is much more toxic than arsenate (
1). Arsenic in the form of arsenilic acid and roxarsone is used in broiler farming to enhance weight gain and to prevent protozoan parasites. If broiler feed contains higher arsenic levels, it may accumulate in the meat and could become a threat to the human health. Clinical signs of arsenic toxicity involve reduced feed intake and weight gain, increased salivation, and general depression (
2). Intermediate metabolites of arsenic are more toxic. These metabolites and/or iron-dependent reactive oxygen species (ROS) elevate protein and lipids membrane peroxidation, resulting in cellular oxidative damage and carcinogenesis in lungs, skin, liver, and bladder (
3). Usually, biotransformation of arsenic occurs in the liver and kidneys (
4).
Imidacloprid (IMI) is one of the most popular broad-spectrum, systemic, neonicotinoid insecticides widely used for the control of sucking, boring, and root-feeding insects (
5). It is also used in many veterinary medicinal products to control fleas in pets and poultry (
6). IMI can cause toxic effects and adverse effects that can be fatal to human and animals (
7). These pesticides can cause oxidative stress generating free radicals and altering the antioxidant and free-radical-scavenging enzyme system (
8). Hematological changes could be observed in chickens exposed to imidacloprid toxicity (
9). Decreased antioxidant activity, increased biomarkers disruption, and liver histopathological changes may be observed after As and IMI exposure (
10).
Prebiotic lactoferrin (LF) is an iron-binding glycoprotein belonging to the transferrin family which plays an important role in regulating microbial activity due to its specific structure. LF is excreted through exocrine glands (breast milk or tears) and mucosae, and it is contained in the secondary neutrophil granules and in the blood (
11). LF has been reported to increase body weight and performance in broiler chicks by enhancing nutrient digestibility and nutrient uptake (
12). It has immune-modulatory roles and could be considered as a part of the body defense mechanism (
13) having antimicrobial and antiviral properties. It acts as activator/modulator of iron-related signalingpathways, reducing inflammatory response, cytokines, and ROS production (
14). Additionally, LF has antioxidant properties (
15).
Numerous studies have demonstrated the antibacterial, antifungal, and anti-parasitic properties of LF, but few have addressed its antitoxic effects. Therefore, this study was designed to investigate the effect of LF feed supplementation on arsenic and/or imidacloprid toxicity in broiler chickens.
MATERIAL AND METHODSEthical statementThis protocol was approved by the Research Committee of the Animal Health Research Institute (c) and authorized by The Institutional Animal Care and Use Committee (ARC-IACUC)/Agricultural Research Center (ARC/AHRI/16/24).
Chemicals and medicationsArsenic (As): Sodium arsenite AR (anhydrous) 99% was obtained from Pioche Laboratory Chemicals Co., Giza, Egypt. It was freshly prepared and administered orally. Imidacloprid (IMI): [1-(6 chloro-3-pyridylmethyl)-N-nitroimidazolidin- 2-ylideneamine], 98.9% technical grade was obtained from Elhelb Pesticides & Chemicals Co, New Damietta–Egypt. It was freshly prepared and administered orally. Lactoferrin (LF): Lactoferrin was obtained from Dulex lab nutrition and pharmaceutical company, New Cairo, Egypt, Batch NO: B2117051.
Vaccines and vaccination protocolAll birds were vaccinated with Newcastle disease virus (NDV) (HitchnerB1 and La-Sota at age of 7 and 18 days, respectively), and with infectious bursal disease (IBD) vaccine at age of 14 days. NDV vaccines were purchased from Intervet Boxmeer Company, Holland. Gumboro vaccine was purchased from Rhone-Merieau Company, France.
Experimental designOne-day-old Cobb broiler chicks (n=105) were obtained from El-Kahera poultry company 10
th of Ramadan City. They were fed on a balanced commercial ration purchased from Feed Mix Company. The birds were acclimatized 7 days before the experiment. On day 8, the chicks were randomly divided in 7 groups (15 each). G1 served as control group and was not given any supplement. G2 was treated orally with As in a dose of 152 μg/kg BW (1/50 of LD50) (
16). G3 was treated orally with IMI in a dose of 5 mg/kg BW (1/20 of LD50) (
17). G4 (As+IMI) was treated orally with As and IMI in the same respective doses. G5 (As+LF) was treated orally with As in a dose of 152 μg/kg BW (1/50 of LD50) and with lactoferrin (250 mg/kg of diet) (
18). G6 (IMI+LF) was treated orally with IMI in a dose of 5 mg/kg BW (1/20 of LD50) and lactoferrin in a dose of 250 mg/kg of diet. G7 (As+IMI+LF) was treated orally with As, IMI, and LF in the same respective doses. The treatment period for all groups was 4 weeks.
Body weight and weight gain were recorded on a weekly interval in a period of 5 weeks.
SamplesTwo blood samples were collected from wing vein at the end of experiment (35
th day). One blood sample was collected in EDTA tube for complete blood count (CBC) (RBCs, Hb, PCV, MCV, MCH, MCHC, Platelets, WBCs, Lymphocytes, Neutrophil). The other blood sample was used for serum separation by centrifugation (3,000 rpm for 5 min). Sera were stored at -20 °C until biochemical analysis.
Following slaughtering, liver, kidney, and muscles samples were obtained for antioxidant, residue detection, and histopathological examination.
Preparation of liver tissue for antioxidants assessment
Liver tissues were cut, weighed, and then minced into small pieces. They were homogenized with glass homogenizer in 9 volumes of ice-cold 0.05 mm of potassium phosphate buffer (pH 7.4), obtaining 10% homogenates. They were centrifuged at 3,000 rpm for 14 min at 4 °C. The supernatant was used for the evaluation of glutathione S-transferase (GST), catalase (CAT), superoxide dismutase (SOD), and malondialdehyde (MDA). Liver tissues (0.3 g) were cut into small pieces and were homogenized by a glass homogenizer in 0.6 ml of 25% met-phosphoric acid (MPA) (ref. No. 253-433-4; Sigma-Aldrich, Germany) and 2.1 ml of distilled water. The homogenate was mixed, incubated at 37 °C for 1 h, and centrifuged for 10 min at 3,000 rpm. The clean supernatant was used for evaluation of reduced glutathione (GSH).
Preparation of arsenic residue sampleThe muscle, kidney and liver samples were thawed at room temperature. A 0.5 g of the homogenized sample was transferred into microwave digestion vessels (XP-1500 Plus). A 7 ml of 65% extremely pure nitric acid was added for 10 min until the yellow fumes dissolved. Hydrogen peroxide (30% H
2O
2) was added in volume of 3 ml. The vessels were sealed and were placed into the microwave digestion system (
19). A clear solution of the digested sample was transferred into 25 ml volumetric flasks which were analyzed for As residue via ICP-MS (Agilent Model: 7700). Blank samples were processed same as the homogenized samples.
Sample preparation for imidacloprid residueIMI residue in muscle, kidney and liver samples were evaluated by HPLC system (
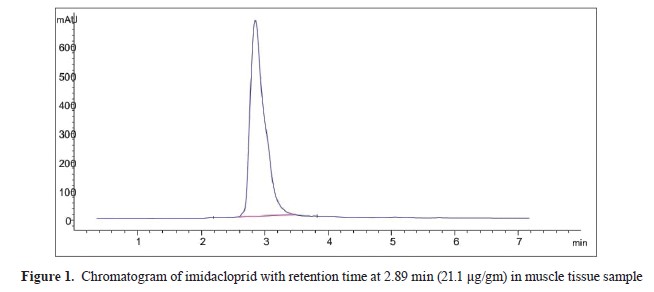
20) (Agilent 1200 series, Software – Agilent Chemi-station) (Agilent Technologies, Germany), with a pump, degasser, auto-sampler, DAD detector, and Agilent C18, 100 A, 4.6×250 mm, and 5 μm Chromatographic column as stationary phase. Standard preparation was performed by a stock solution in isoctan that was used for an intermediate solution preparation (100 mg/L). Working standards were prepared by using the intermediate solution in blank, muscle, kidney, and liver (control group) in IMI range of 10-10,000 μg/L. The standard curve had a good linearity (r2≥0.9996). The samples were digested by using 1 g of tissue which was cut with scissors and then homogenized in a Remy-tissue homogenizer (RQ-127A) with 5 ml of acetonitrile for 4 min. The homogenized samples were filtered through filter paper (Whitman No. 1). The sample residues were re-homogenized and re-extracted twice with 5 ml of acetonitrile and filtered via Whitman No. 1 filter paper. The acetonitrile filtrates were transferred to a clean, dry separator funnel, and n hexane was added in 1:1 ratio. The mixture was shaken vigorously for 2 min and was settled for another 2 min until both phases were clearly separated. The lower acetonitrile phase was collected in another separator funnel and re-partitioned twice by n-hexane. The acetonitrile phase was separated in another conical flask and evaporated by using a rotary vacuum evaporator. Finally, the volume up to 1 ml was adjusted with acetonitrile (HPLC grade). The clear sample was filtered through a 0.20 μl membrane filter before injection into the HPLC, with a retention time of 2.89 min for IMI (
Fig. 1).
 Histopathological examination
Histopathological examinationLiver samples were fixed in 10% buffered neutral formalin solution for 24 h, dehydrated in gradual ascending ethanol, cleared in xylene, and embedded in paraffin wax. Paraffin tissue sections (5 μm) were sliced with a microtome (Leica RM 2155, England), stained with hematoxylin and eosin (H&E), and were examined microscopically (
21).
Hematological and biochemical analysisErythrocyte blood count (RBC, 106/ml), hemoglobin concentration (Hb, g/dl), white blood cell count (WBC, 10
3/ml), platelets count (PLT, 103/ml), and packed cell volume (PCV, %) were evaluated according to Feldman et al. (
22). Two blood-smears were prepared immediately after theblood collection. Stained blood films by Giemsa were used for differential leucocyte count according to Anderson and Latimer (
23). Liver status was assessed by alanine amino transferase (ALT), aspartate amino transferase (AST), γ-glutamyltransferase (γGT), and alkaline phosphatase (ALP) serum levels determined by spectrum kit (CAT. No 265 001, 260 001, 246 005, and 215 002, respectively). Kidney status was assessed by urea, creatinine, and uric acid serum levels determined by spectrum kit (CAT. No 319 005, 235 004, and 323 000, respectively). Additionally, total antioxidant capacity (TAC) and nitric oxide (NO) were determined by using Bio-diagnostic kit CAT.
No. TA 2513 and No. 25 33, respectively. Liver tissues were analyzed for MDA, CAT, GSH, SOD, and GST levels measured with Bio-diagnostic kit CAT. No. MD 25 29, CA 25 17, GR 25 11, SD 25 21, and GT 25 19, respectively. Serum total protein and its electrophoretic patterns (total protein, albumin, globulin, A/G ratio, alpha 1, alpha 2, beta 1, beta 2, gamma 1, and gamma 2) were assessed with Spectrum kit CAT. No. 310 001 and polyacrylamide gel electrophoresis according to Davis (
24). The electrophoresis patterns were calculated according to SynGene S. No. 17292*14518 sme*mpcs.
Statistical analysisThe data were presented as mean ±standard error. The values were compared by One-Way ANOVA (SPSS version 22). The level of significance was set at p<0.05.
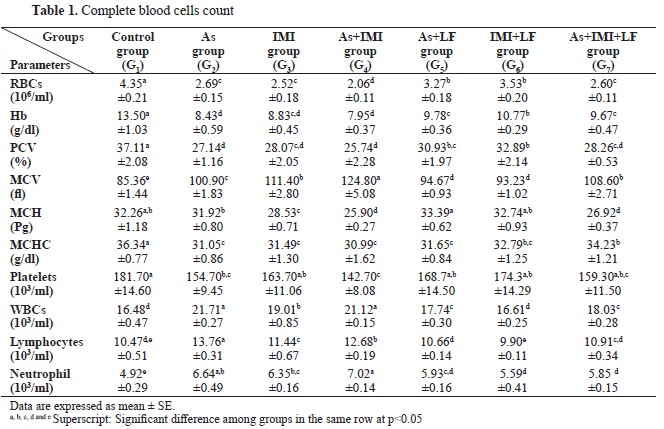
RESULTSThe CBC revealed a significantly lower RBC, Hb, PCV, MCHC, MCH, and platelets count in groups G2, G3, and G4, respectively. Significantly higher values were observed for MCV, WBC, lymphocytes, and neutrophils compared to the control group (
Table 1). The groups treated with LF (G5, G6, and G7) showed significantly higher values for the same parameters compared to G2, G3, and G4.
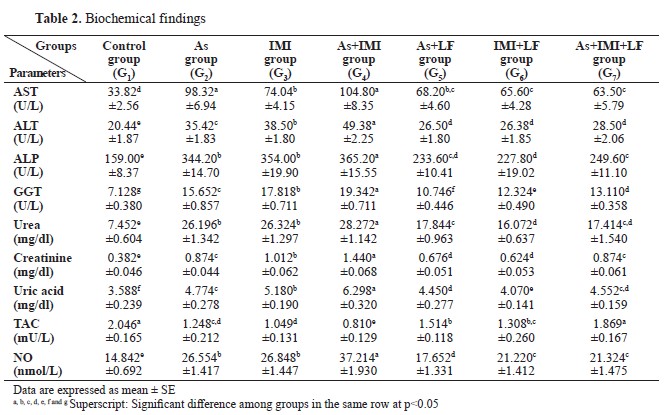
There were significantly higher values for AST, ALT, ALP, GGT, urea, creatinine, uric acid, and NO levels, and significantly lower for TAC in G2, G3, and G4 compared to the control. G5, G6, and G7 groups treated with LF showed significantly lower AST, ALT, ALP, GGT, urea, creatinine, uric acid, and NO levels, and significantly higher levels of TAC compared to G2, G3, and G4 (
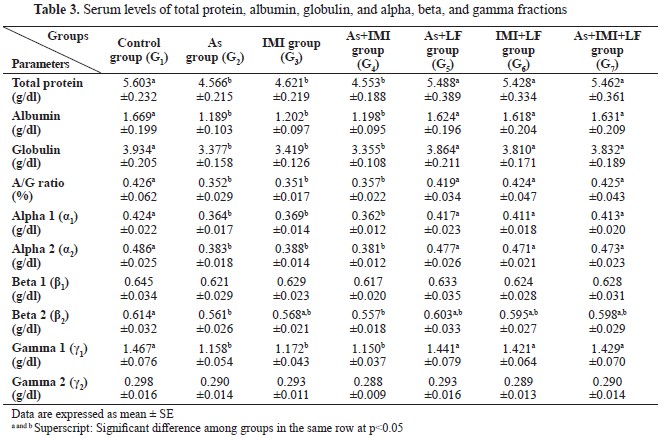
Table 2). G2, G3 and G4 had significantly lower values for total protein, albumin, globulin, A/G ratio, alpha 1, alpha 2, and gamma 1 compared to the control, G1, or LF-treated groups G5, G6, and G7. The control had significantly higher levels of beta 2 compared to G2 and G4, but non-significantly higher than the other groups. Beta 1 and gamma 2 were non-significantly different between the groups. LF-treated groups (G5, G6, and G7) showed non-significant differences in total protein, albumin, globulin, A/G ratio, alpha 1, alpha 2, and gamma 1 levels compared to the control group (
Table 3).
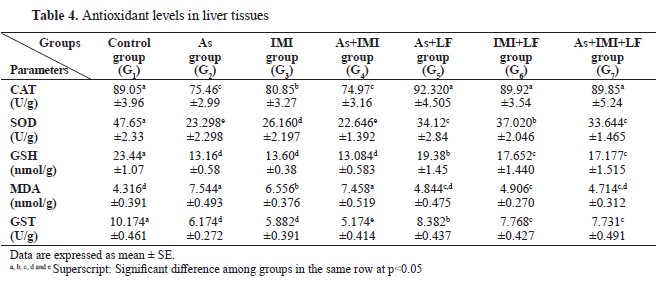
CAT, SOD, GSH, and GST levels were significantly lower, whereas MDA was significantly higher in G2, G3, and G4 compared to the control. LF-treated groups G5, G6, and G7 had significantly higher CAT, SOD, GSH, and GST levels, and significantly lower MDA level compared to G2, G3, and G4 (
Table 4).
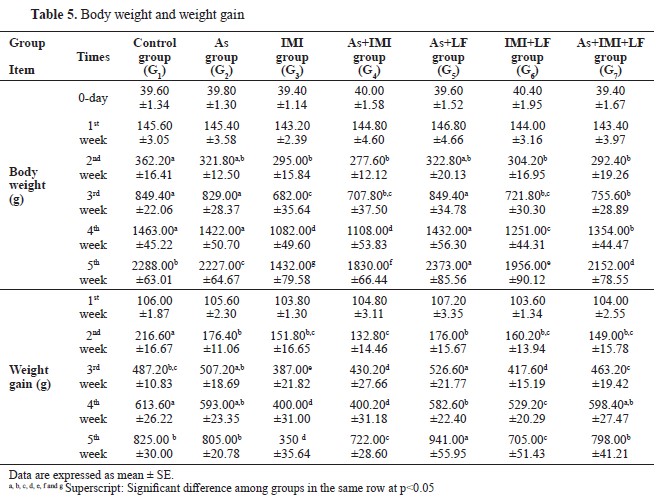
Body weight and weight gain were significantly lower in G2, G3, and G4 compared to the control. LF-treated groups G5, G6, and G7 had significantly higher body weight and weight gain compared to the other groups treated with As and/or IMI. This was more pronounced in the last week of the experiment (
Table 5).
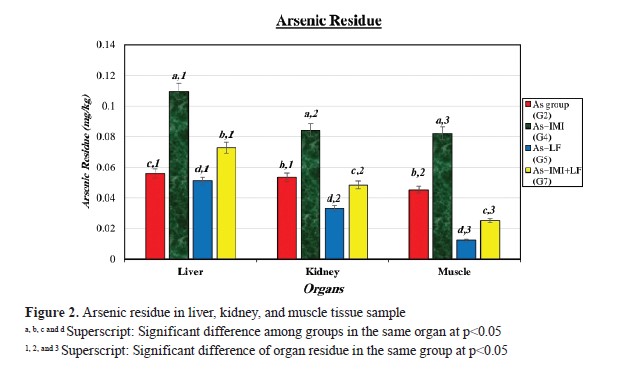
Liver tissue samples from the experimental groups had As residues (
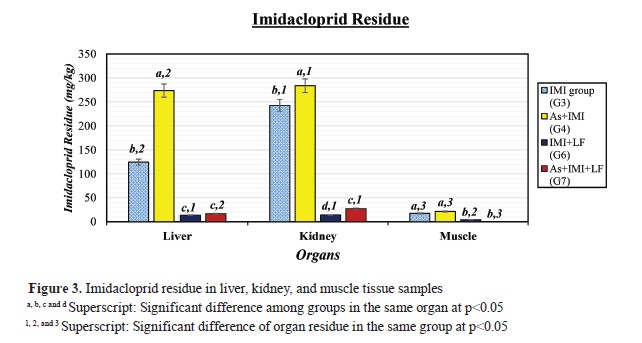
Fig. 2). G4 had higher As r esidues i n a ll o rgan s amples. G 2 a nd G 4 had higher As residue levels in liver, kidney, and muscle samples, while LF-treated groups (G5 and G7) had lower. IMI residues were detected in the kidney samples of the experimental groups (
Fig. 3). The highest IMI level was in G4, while, LF-treated groups (G5 and G7) had significantly lower residues in all organ samples. G7 had lowest IMI residue in muscle tissue samples.
Liver histopathology revealed normal histological appearance of hepatic acini, portal areas, and central veins in the control group (G1) (
Fig. 4-A, B). G2 had numerous vacuolated hepatocytes, congested blood vessels with edematous wall and hyperplastic changes within bile ducts (
Fig. 4-C, D). G3 had focal necrotic areas, severe congestion in most hepatic blood vessels, hyperplasia of bile duct epithelium with destruction of the lining epithelium (
Fig. 4-E, F).
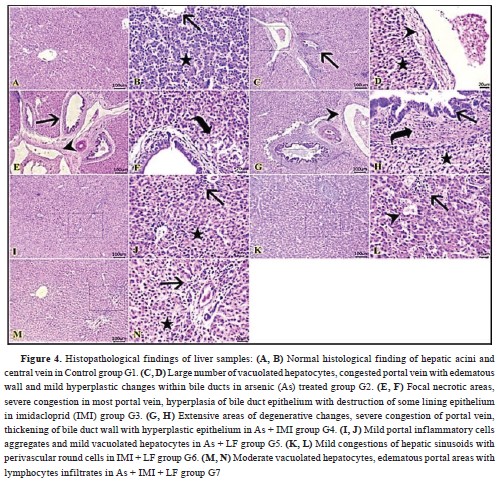
Extensive areas of degenerative changes severe congestion of portal veins, chronic cholangitis with hyperplastic epithelium and bile stasis were observed in G4 (
Fig. 4-G, H). G5 had mild portal inflammatory-cell aggregates and mildly vacuolated hepatocytes (
Fig. 4-I, J). Mild congestions of hepatic vessels with perivascular round cells were observed in G6 (
Fig. 4-K, L). Moderate vacuolated hepatocytes, edematous portal areas with lymphocytic infiltrates were seen in G7 (
Fig. 4-M, N).
DISCUSSIONThe current study aimed to assess the effect of LF in reducing the As and IMI induced changes on immunological and serum biochemistry parameters in broiler chicks. The decreased RBC, Hb, and PCV values in G2, G3, and G4 were consistent with similar studies in chicken exposed to As (
25) and in broiler chicks exposed to IMI (
9, 26). Metal and insecticide induced toxicity on erythrocytes is exerted by oxidative damage that could lead to anemia and hypoxia. They can have direct effect on the bone marrow, liver, and kidney thus altering hemopoiesis and erythropoietin production (
2). This effect was more highly expressed in animal groups that were treated with more than one toxin.
Hematological parameters were higher in LF-treated groups. This was confirmed in another study with iron-rich LF-supplemented diet which improved anemia in treatment groups. This suggests that the animal’s physiological state and the availability of iron are contributing to the LF’s ability to improve hematological indices (
27). Elevated WBC, lymphocytes, and neutrophil levels in G2, G3, and G4, may be related to the toxins-induced leucopoiesis.
However, they may exert immunosuppressive properties as well (
9). This type of leukocytosis was recorded in chickens exposed to inorganic As (
25). Arsenic can cause immunosuppression by altering the activity of lymphocytes, macrophages, and monocytes (
28). The LF capacity to alleviate the negative effects of toxins on blood cells indicates its’ regulatory role on the immune system (
15).
The significantly lower total protein, albumin, and globulin levels in G2, G3, and G4 were similar to the findings in chicks exposed to IMI (
26). The reduced serum protein content could be explained with the negative effect of IMI on hepatic protein synthesis at post-transcriptional level via competitive inhibition of phenylalanine t-RNA synthesis resulting in inhibition of amino acylation and peptide elongation (
29). IMI impairs amino acid gut absorption which contributes additionally to the lowered protein profile (
30). Heavy metals such as As also have a carcinogenic effect and causes liver damage leading to inhibition of hepatic protein synthesis. Arsenic toxicity in birds was observed with decreased serum proteins due to reduced feed intake and increased protein catabolism. It also causes extensive damage to capillaries resulting in increased permeability (
2). Increased liver enzymes with concomitant proteolysis could additionally contribute to decreased serum proteins (
31). Reduced albumin levels could be used as an indicator of glomerular and mucus membranes destruction. This is mostly related with the As potential to accumulate in the liver and kidneys (
4).
Serum ALT, ALP, AST, γGT, urea, creatinine, and uric acid levels were significantly higher in G2, G3, and G4 compared to the control. These findings are similar with studies conducted in mice (
32) and in chickens exposed to IMI (
26, 33). The increased liver and kidney enzymes might be related to the hepato-renal damage induced by As and IMI due to enhanced cell membrane permeability. Liver damage observed in the current study may be related to increased ROS production during the As and IMI metabolism which was observed with highly decreased TAC and significantly reduced antioxidant enzymes (
10). Increased ALP and ALT might be attributed to As toxicity on liver, intestines, bile duct epithelium, and hepatocytes. ALP elevation could also be related with the disruption of the hepatic flow due to cirrhosis and enzyme reabsorption into the blood (
34). High level of urea, creatinine, and uric acid were related with the biotransformation of arsenic in the kidneys and the concomitant renal and glomerular failure (
4). Conversely, LF-treated group had significantly different values for these biomarkers compared to the groups treated with As and IMI. These findings are confirmed with another report where LF is attributed to support hepato-renal functions through the reduction of protein loss, tissue damage, and enzyme release (
15). Serum albumin, alpha-, beta, and gamma- globulins are considered as reliable diagnostic indices. The LF-treated chicks in this study had elevated levels of gamma globulins. Therefore, LF could be considered with hepatoprotective and immunomodulatory properties (
13).
The lower TAC, CAT, SOD, GSH, and GST, and higher NO in the serum and liver samples in the group that was treated with As and IMI was similar to the results recorded in chicks exposed to IMI (
26). Decreased SOD, CAT, and GSH, with increased MDA, were recorded in chickens exposed to inorganic As in other studies (
25, 34). These studies confirmed that the oxidative stress was related with As-toxicity due to overproduction of free radicals and depletion of the antioxidant enzymes (
35). This leads to increase in lipid peroxidation products (MDA) (
10). Additionally, the combined As and IMI administration caused a greater decrease in liver antioxidant enzymatic activities compared to a single administration of each (
36). The elevated MDA in the current study indicate on decreased cell membrane integrity and cellular functions (
37). The higher antioxidants activity in the groups administered with As and/or IMI, and treated with LF, may be attributed to its role to reduce the levels of intracellular ROS and MDA and to regulate cellular antioxidants enzymes. Additionally, increased CAT and SOD activities may be associated with the ability of LF to reduce lipid peroxidation by iron sequestration (
15) and with its iron-binding properties. Hence, these enzymes require the iron-sulfur cluster and ironprotoporphyrin as a cofactor (
13).
The decreased body weight and weight gain observed in the groups, administered with As and IMI, were in compliant with the studies in chickens fed with a diet contaminated with IMI (
9, 33). They concluded that IMI had adverse effects on general health status and growth performance. The decreased body weight may be due to the high oxidative instability leading to low nutritional intake and decreased metabolism in chickens. As-toxicity in broiler chicks may lead to general depression, decreased body weight, feed intake, and feed conversion (
38). Higher body weight and weight gain observed in LF-treated groups was previously reported in other similar studies (
15, 18). LF enhanced nutrient uptake and digestibility (
11, 12) even during As and IMI exposure.
The withdrawal period of a medication is the time elapsed between the administration and its concentration fall to the approved level. This period guaranties that food from animal origin does not contain medications above the maximum residue limit. In this study, the repeated oral supplementation of IMI and As has significantly increased their residue concentrations in liver, kidney, and muscles. The accumulation of IMI and As in different tissues is a good indicator for their absorption in chickens (
39). Our findings are in accordance with previous studies in rabbits which reported As accumulation in different organs, with higher tendency for the liver compared to kidney and muscles (
40). The As biotransformation generally occurred in liver and kidneys (
4). The highest accumulation was recorded in groups that were administered both with As and IMI because the latter potentiates As toxicity and tissue accumulations (
10). Again, LF-treated groups had lower toxin residues due to its modulating effects on As and/or IMI.
The liver changes in the current study were recorded with necrosis, congestion, inflammatory cellular aggregates, and edema in the portal triad. Similar findings were reported in chicks exposed to As (
34) and in mice exposed to IMI (
32). The histopathological findings in the LF-treated group showed mild amelioration in hepatic tissue changes compared with the groups that were administered with the toxins. This confirms the LF role in modulating the toxicants by preventing ROS accumulation and subsequent oxidative damage.
CONCLUSIONToxicity of As and/or IMI adversely affects CBC, serum biochemistry, liver antioxidant activity and body weight with concomitant histopathological changes in the liver tissue of broiler chicks. IMI potentiates As toxicity and tissue accumulations. LF is capable to modulate As and/or IMI toxicity and to reduce IMI-induced oxidative stress in chickens by increased cellular antioxidant activity. Furthermore, LF may be used as natural growth stimulant in broiler chickens as it has positive effect on growth performance even during As and/or IMI administration.
CONFLICT OF INTERESTThe authors declare that they have no known conflict of interest in the conduction of the current study.
ACKNOWLEDGMENTSAuthors are greatly thanked to Dulex Lab Nutrition & Pharmaceutical Company, New Cairo, Egypt and El-helb Pesticides & Chemicals Co., New Damietta, Egypt for providing us with Lactoferrin raw material and imidacloprid respectively. We would like to thank Dr. Ahmed M. El-Mahdy, Pharmacology and Pyrogenic Unit, Department of Biochemistry, Toxicology and Feed Deficiency, Animal Health Research Institute (AHRI), Dokki, Giza on his valuable help in the analysis of imidacloprid tissue residues.
AUTHORS’ CONTRIBUTIONAll authors participated in research design and sample collection. MKM, AGAE, NFE, SME, HMY and MFH performed the blood and serum biochemical analysis of experiment. SMS performed the histopathological examination. MKM and HMY performed the data analysis. All authors participate in writing and approved the final version of the manuscript.

 10.2478/macvetrev-2024-0028
10.2478/macvetrev-2024-0028








