As an endemic disease of some hot climates with global distribution, spirocercosis is caused by the parasitic nematode
Spirocerca lupi. The disease manifests itself with varying clinical presentations depending on the stage of the disease, aberrant migrations, and possible complications. The diagnosis of the disease is confirmed by the detection of normal embryonated eggs in stool exam or parasites in esophageal nodular lesions (
1).
The nematode infects carnivores through the ingestion of infected dung beetles or a variety of paratenic hosts, including amphibians, reptiles, lizards, domestic and wild birds, and small mammals, such as hedgehogs, mice, and rabbits. Then, the larvae penetrate the gastric mucosa and start a predictable migration path along the arteries, completing their life cycle in the thoracic aorta. About 3 months after the ingestion of the larvae, they leave the aorta and migrate to the distal esophagus, where they induce nodular lesions and become mature parasites within three months (
2, 3). As time passes, about 25% of the nodules caused by the parasites undergo neoplastic changes, leading to fibrosarcomas, osteosarcomas, or anaplastic sarcomas (
4). The characteristic clinical manifestations associated with spirocercosis are linked to esophageal nodules and encompass regurgitation, emesis, dysphagia, and weight reduction. Aberrant migration of
Spirocerca lupi has been documented in nearly all thoracic organs, as well as the gastrointestinal and urinary systems, in addition to subcutaneous tissues (
5).
Spirocerca lupi instigates the development of nodular formations within the submucosal and muscular layers of the esophagus and possesses the capability to migrate through the aorta, resulting in ossifying spondylitis of the thoracic vertebrae and the formation of aneurysms. Aberrant larval migration of this nematode into the central nervous system may cause neurological disturbances (
6). Non-neoplastic
S. lupi induced esophageal nodules are often misidentified as granulomas. Nonetheless, granulomas are characterized by organized macrophages and granulation tissue, whereas
S. lupi nodules are primarily composed of neutrophils and B and T lymphocytes, lacking granulation (
7). Chronic inflammation is the most accepted factor for parasite-induced carcinogenic mechanisms. Accordingly, a relationship between inflammation and neoplastic progression has been postulated for
S. lupi, Heterakis spp
., T. taeniformis and liver flukes. Furthermore, inflammation, as a cancer promotor factor, can cause DNA damage and post-translational changes, including physical injuries, oxidative stress, inflammatory cytokines, prostaglandins, and growth factors (
8). Other mechanism of carcinogenesis caused by
S. lupi and other canine carcinogenic nematodes is the permanent inflammation induced by the release of
S. lupi-derived Excretory and Secretory Products (Sl-ES) in canine tissues (
8, 9). It is assumed that these molecules contribute to carcinogenesis with a complex interaction between host cells and the parasite by accelerating and regulating the endogenous parasite-related inflammatory process (
8). However, there are limited studies on the role of growth factors in the pathogenesis of canine spirocercosis. According to the studies, the spirocercosisrelated non-neoplastic nodules and sarcomas show overexpression of Vascular Endothelial Growth Factor (VEGF), Fibroblast Growth Factor (FGF), and Platelet-Derived Growth Factor (PDGF) in Immunohistochemical (IHC) investigations (
10). As a member of the Transforming Growth Factor β (TGF-β) superfamily, the Glial cell linederived Neurotrophic Factor (GDNF) is effective in the development of the neurons of the Central Nervous System (CNS) and other cells outside of the CNS, such as skeletal and smooth muscle cells, spermatogonia, and renal cells (
11, 12). Moreover, the presence of GDNF has been confirmed in monocyte-derived cells, such as macrophages and microglia (
11), while its overexpression has been reported in some developing cells, tissue injuries, and disorders like enteric parasitic diseases, Benign Prostatic Hyperplasia (BPH), and neural injuries (
11, 13). Considering the overexpression of GDNF in enteric infection with
Hymenolepis diminuta, it has been assumed that it has a neuroprotective, neuro-restorative, smooth muscle-changing, and enteric neural system-plasticizing role in enteric infection with tapeworms (
14). It has been shown that p53 has an essential role in cell cycle arrest and regulation of the apoptosis pathway, which is induced by several factors, such as cleaved Caspase 3. Damage to DNA triggers the stabilization, activation, and phosphorylation of p53, similar to other immunological killing processes (
15). Thus, as a tumor suppressor gene, p53 controls cellular proliferation and triggers apoptosis in malignant cells (
16). On the other hand, Ki67, as an IHC marker, is used for evaluating cellular proliferation rate, especially in canine Soft Tissue Sarcomas (STSs) (
17). This marker is expressed in all phases of the cell cycle, with the highest during mitosis. However, its detection during G0 is impossible due to its short half-life (
18). It has been confirmed that p53 and Ki67 proteins can be used for detecting precancerous tissues. Previous studies have shown that p53 accumulation depends on the mutations in its gene, which happens during the early steps of esophageal carcinogenesis (
19). Moreover, the Terminal deoxynucleotidyl Transferase (TdT)-mediated dUTP Nick End Labeling (TUNEL) is an extensively used method for detecting apoptotic cells by labeling DNA fragmentations (
20). It seems that the induction of apoptosis in inflammatory immune cells, especially lymphocytes, has an immunosuppressive effect in parasitic diseases. Accordingly, parasite-induced apoptosis of T cells weakens cell-mediated immunity (
21).
The present study aimed to evaluate the expression of GDNF as a neurotrophic factor and p53 as a cell cycle regulator in canine esophageal tissue containing non-neoplastic nodules induced by
S. lupi. Ki67 levels were assessed to determine the rate of cellular proliferation, while the TUNEL test was used to detect apoptosis. Additionally, the study aimed to determine the ratio of cellular proliferation/apoptosis in these parasite-induced nodules and to classify the inflammatory cells in the parasitic nodules with CD3 (for T cells), CD20 (for B cells), and CD68 (for macrophages) markers.
MATERIAL AND METHODSAnimal group assignmentThe present study included 152 Iranian mixedbreed dogs examined from June 2017 to June 2022 in Urmia, Iran (Urmia city located in West Azerbaijan province of Iran). The dogs included 87 male (57.2%) and 65 female (42.8%) dogs. Moreover, 35 dogs (23.0%) were younger than 3 years of age, 64 dogs (42.1%) were aged 3-6 years, and 53 dogs (34.9%) were older than 6 years of age. Additionally, 55 (36.2%), 34 (22.4%), 41 (27.0%), and 22 (14.5%) dogs were examined in spring, summer, autumn, and winter, respectively.
The animals underwent clinical examinations at a veterinary hospital for the manifestations of
S. lupi, such as dysphagia and megaesophagus. The EDTA-containing blood samples obtained from the jugular vein of 152 examined dogs using a 21-gauge needle, were evaluated by an Abbott Cell Dyn 3500 automated hematology analyzer. Stool samples of the dogs were evaluated using the Telemann concentration stool exam to detect the parasites’ eggs based on the method by Mylonakis et al. (
22). Briefly, 1 g of feces was completely mixed with 6 mL of HCl and then filtered using a 1 mm mesh. Afterward, 6 mL of diethyl ether was immediately added to the suspension, and the suspension underwent shaking for 30 sec and was centrifuged for 1 min. The supernatant fluid was then cast off, a few drops of the sediment were placed on a glass slide and covered with a coverslip. The slide underwent microscopic examination to find the barrelshaped, thick-walled eggs of
S. lupi. Esophageal tissues were collected from the necropsied dogs (n=64), of which 44 were with normal findings and 20 were with nodular changes. However, 20 normal and 20 nodular tissues were considered for histopathological and immunohistochemical examinations. The present study was conducted and approved under the supervision of the Research Ethics Committees of the Islamic Azad University, Urmia Branch, with the approval number IR.IAU. URMIA.REC.1401.028 on April 24, 2022.
Preparation of histopathologic slidesA total of 20 normal esophageal samples, which were randomly selected, and 20 esophageal samples containing parasite-induced nodules were fixed in 10% buffered formalin, followed by dehydration, clearing, impregnation, and embedding. Then, paraffin blocks containing tissue samples were cut into 6-μm-thick sections using a rotary microtome (Leica RM2125 RTS, Germany), and the sections underwent Hematoxylin & Eosin (H&E), Periodic Acid Schiff (PAS), and Masson’s trichrome staining procedures.
ImmunohistochemistryThe EnVision®+ Dual Link System HRP was used for IHC staining. The xylene dewaxed sections underwent rehydration with ethanol and the slides were incubated in hydrogen peroxide (3%) to inhibit the endogenous peroxidase activity (30 min). Antigen retrieval was performed with 0.01 M citrate buffer (pH 6.0) for 25 min, and the slides were incubated in bovine serum albumin (5%) in Triss-Buffered Saline (TBS) to block the non-specific stains for 30 min. Then, the slides were washed in water and TBS, and were incubated in a normal room temperature. The slides were then incubated overnight at 4 °C in primary antibodies, including GDNF (Santa Cruz Biologicals, Dallas, Texas, USA, 1:150), p53 (Dako, Glostrup, Denmark, 1:100), Ki67 (Dako, Glostrup, Denmark, 1:150), CD3 (Dako, Glostrup, Denmark, 1:100), CD20 (Dako, Glostrup, Denmark, 1:200), and CD68 (Dako, Glostrup, Denmark, 1:25). Finally, the slides were rinsed with Phosphate-Buffered Saline (PBS) and treated with streptavidin-horseradish peroxidase for 20 min. The slides were rinsed with a buffer and treated with diaminobenzidine for 10 min, and the sections were counterstained with Hariss hematoxylin (13). The suitable tissues were used as the positive controls including brain, appendix, and spleen tissues for GDNF, Ki67, and CD68 and tonsil tissue for p53, CD3 and CD20. On the other hand, the sections prepared with nonimmune IgG instead of primary antibodies, were considered the negative controls. Afterward, the immunohistochemical scoring was conducted according to Dvir et al. (
4), and Dvir and Clift (
10). Accordingly, to evaluate positive immunoreactive cells and the expression levels in each section, 5 random microscopic fields with 40× magnification were selected. Each field included at least 200 cells, making a total of 1,000 cells in 5 fields, and the fields did not overlap. The sections without or with scant immunopositive cells were scored 0, those with obvious positive cells, but not in all fields (mild immunoreaction), were scored 1, those with positive cells in all fields that were not dominant (moderate immunoreaction), were scored 2, and those with positive cells, dominant in all fields (severe immunoreaction), were scored 3.
TUNEL stainingThe TUNEL assay (Roche Molecular Biologicals, Basel, Switzerland) was used to detect apoptosis by DNA fragmentation as follows: In brief, the 5-6-μm thick sections were deparaffinized three times (each time 5 min) with xylene, were rehydrated with 70-96% alcohols with a descending gradient (3 min for each gradient), and rinsed in distilled water. Next, the proteinase K (10-20 μg/mL in 10 mM Tris/HCl, pH 7.4-8) was added to the slides, followed by incubation at 37 °C for 15 min, and triple washing with PBS. The sections were incubated with 25 μL of TUNEL solution in a humidified atmosphere at 37 °C for 60 min, and were washed in PBS. The slides were then covered with 25 μL of POD convertor, incubated for 30 min at 37 °C, and washed with PBS three times. Finally, the sections were incubated with about 25 μL of DAB substrate for 60 sec, triple washed with distilled water, and counterstained with hematoxylin (
23). Depending on the specific model being examined, the categorization and evaluation of cellular apoptosis on an ordinal scale of minimal, mild, moderate, and severe, was considered as adequate (
24).
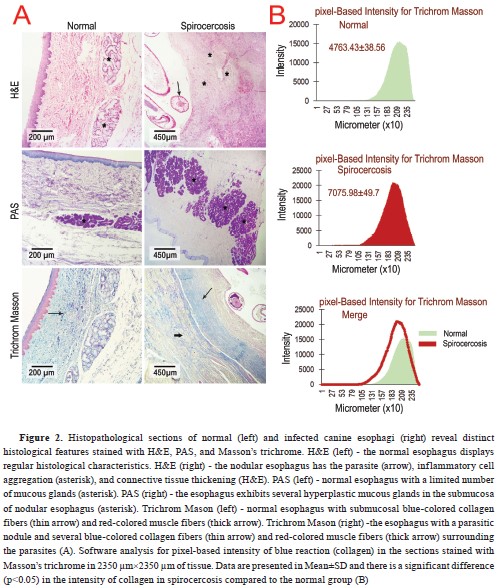
Microscopic tissue sections’ imagingExamination of the histopathological and immunohistochemical tissue sections was performed by using a light microscope (Nikon, Japan), equipped with an ApoTome optical sectioning device and SONY on-board camera (Zeiss, Cyber-Shot). The figures were compiled using Adobe Photoshop CS10 (Adobe, Mountain View, California, USA). A pathologist examined all the histopathological and immunohistochemical sections in a blinded fashion. The positive reactions were evaluated by analyzing the cross-sectional photomicrographs with ImageJ software (Media version: 6.00, National Institutes of Health, USA). In this respect, cross-sectional 20-megapixel photos were taken and subsequently, the pixel-based intensities of blue-stained (representing collagen in Masson’s trichrome) per total pixels were evaluated in a 2330 μm×2330 μm photomicrograph. In IHC and TUNEL stained tissue sections, the pixel-based intensity of positive immunoreactions was analyzed by the software as the mean (±SD) of positive immunoreactivities (brown/total pixels) in 2350 μm2 of each photomicrograph from one section (5 random microscopic fields/each sample). Also, according to Devir and Clift (
10), a pathologist blindly counted the numbers of Ki67+ cells and TUNEL+ cells in various tissue components (epithelium, connective, muscular, and mucous gland) of different groups.
Statistical analysisThe quantitative data were presented as mean ± Standard Deviation (SD), while the qualitative data were presented as frequency and percent (%). For quantitative data, the assessment of normality was conducted with the Kolmogorov-Smirnov test. Subsequently, the data’s homogeneity of variance was evaluated with the Levene’s test. Based on the normal distribution of the quantitative data, the T-test was executed to analyze the results. The student T-test and Chi-square test were used for comparative analysis. The Bonferroni post hoc test was used in case of significant intergroup differences. Finally, the Mann-Whitney U test was used to compare the qualitative data. The significance level was set at 0.05, and statistical analysis was performed with IBM SPSS software version 26.
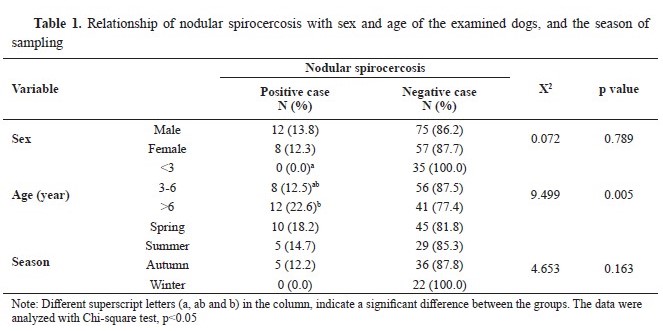
RESULTSA total of 20 out of 152 dogs (13.6%) were diagnosed with nodular spirocercosis based on clinical findings, hematological investigations, and necropsy. Moreover, 16 out of 152 dogs (10.52%) had positive stool exams for
S. lupi. Thus, 31 out of 152 dogs received a confirmed diagnosis of spirocercosis. All necropsied cases had positive stool exams. There was no significant relationship between spirocercosis occurrence with gender and season (p>0.05). However, spirocercosis had a significant relationship with age, showing the highest prevalence in the age group of 3-6 years (p<0.05) (
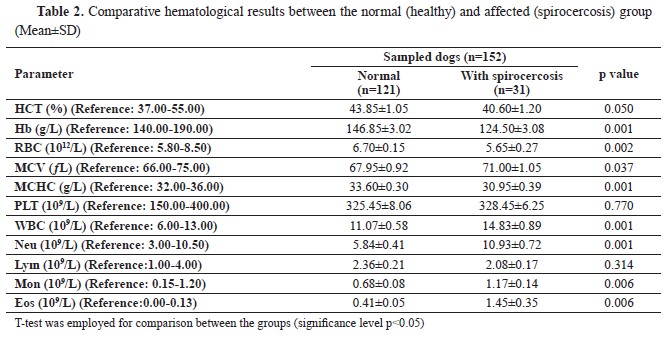
Table 1). Finally, there were significant relationships between spirocercosis and all hematological parameters (p<0.05), except for the lymphocyte and platelet counts (p>0.05) (
Table 2).
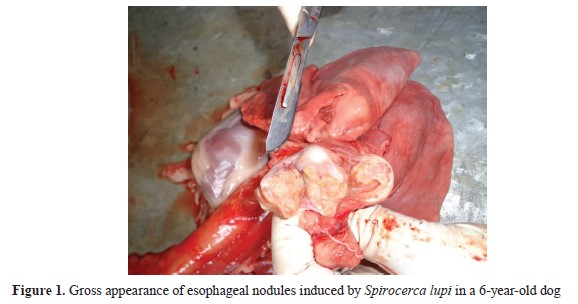
Nodular spirocercosis was diagnosed in 20 dogs. The parasite-induced non-neoplastic nodules were generally observed in the lower third of the esophagus. The nodules had a size of 3-23 mm, and most of them included a 3.0-6.5-cm parasite surrounded by pus (
Fig. 1).
The H&E-stained slides prepared from the nodules showed a thickened squamous epithelium compared to the normal esophageal epithelium. Moreover, the parasites observed in the central area of the nodules were surrounded by inflammatory cells, including neutrophils, lymphocytes, and macrophages, that were aggregated among collagen and muscle fibers (
Fig. 2A). All of the parasitic nodules had the combined characteristics of early inflammatory and pre-neoplastic nodules (simultaneous increase of fibrocytes and collagen fibers with inflammatory cell infiltration). In the PAS-stained slides, a mucous gland hyperplasia (without any atypia) has been found (
Fig. 2A), while the slides stained with Masson’s trichrome, showed an increased number of collagen fibers (blue-colored) and muscle fibers (red-colored) in the nodules compared to the normal esophageal tissue (
Fig. 2A and 2B).
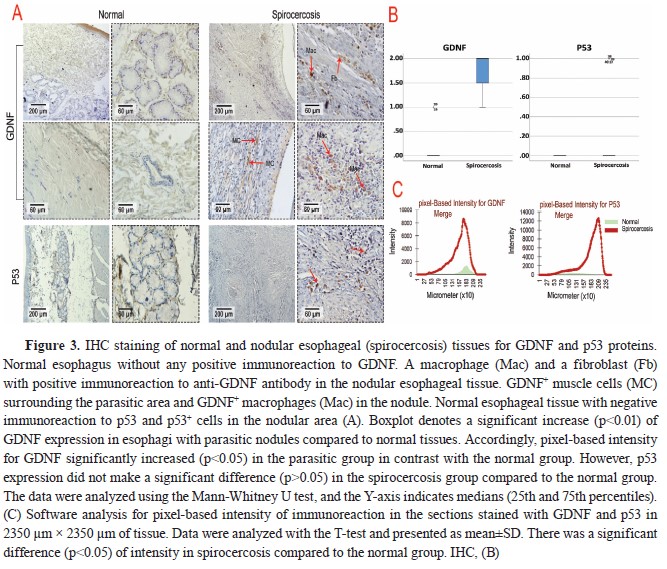
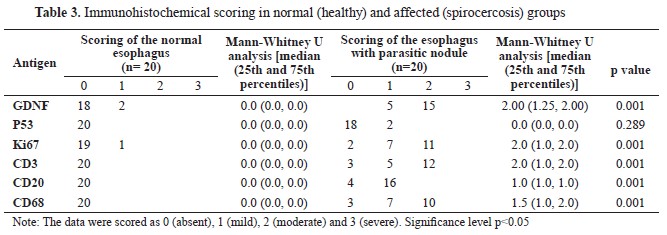
According to the results of the IHC scoring for GDNF, p53, Ki67, CD3, CD20, and CD68 in Table 3, the cellular components of the esophageal tissue, including fibroblasts, muscle cells, and macrophages, showed significant overexpression of GDNF in the infected dogs compared to the healthy dogs, which showed very weak immunolabeling for GDNF in their esophageal tissue (
Fig. 3A). Moreover, GDNF had significantly higher (p<0.01) expression in esophagi with parasitic nodules compared to normal tissues. Accordingly, pixel-based intensity for GDNF significantly increased (p<0.05) in the parasitic group in contrast with the normal group (
Fig. 3C). P53 expression was also mildly positive in only two esophageal tissues of the infected dogs but was entirely negative in the healthy dogs (
Table 3,
Fig. 3A). The p53 expression was not significantly different (
Table 3), despite the pixel-based intensity only in two parasitic esophagi (
Fig. 3B).
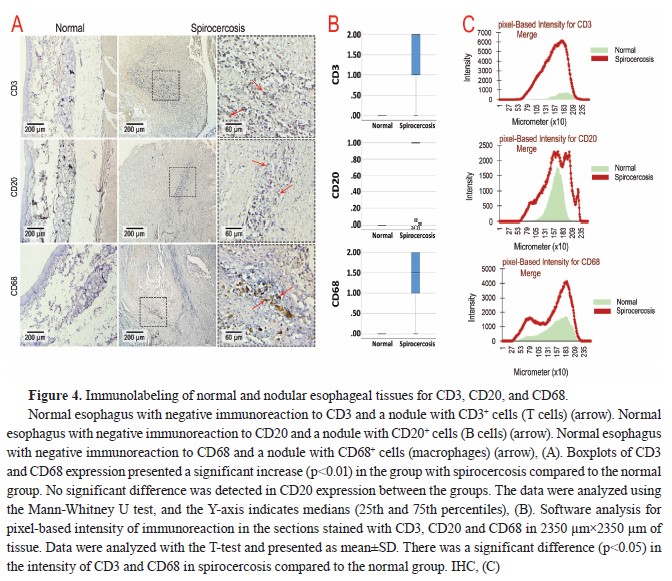
On the other hand, IHC studies showed that neutrophils were the most abundant cells in the nodules, followed by T cells (CD3
+), B cells (CD20
+), and macrophages (CD68
+), while the expression of the markers related to immune cells was negative in normal esophageal tissue (
Table 3 and
Fig. 4A). Accordingly, the T-test analysis results for CD3 and CD68 expression showed a significant increase (p<0.01) in the group with spirocercosis compared to the normal group (
Fig. 4B). However, no significant difference was detected in CD20 expression between the groups (
Fig. 4B).
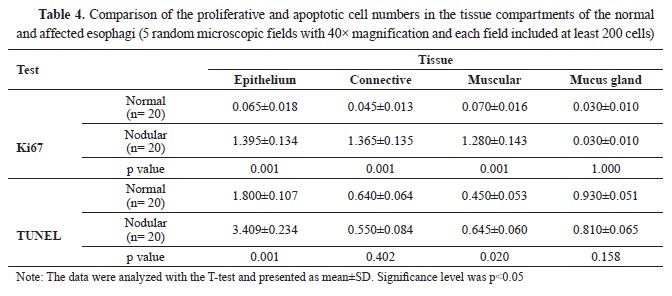
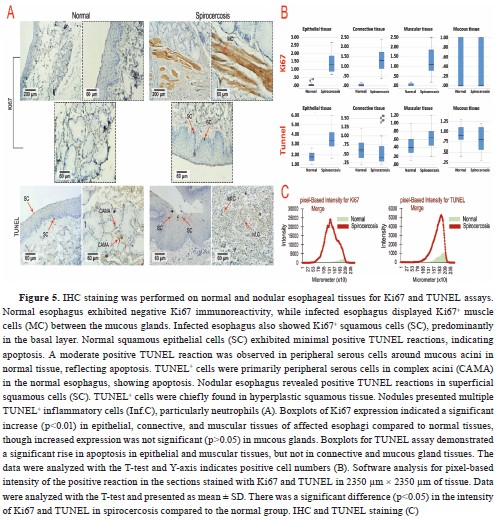
Manual cell counting by a pathologist indicated that the mean number of Ki67 immunolabelled epithelium, muscular, and connective tissue cells in parasitic animals was significantly increased (p<0.05). At the same time, there was no significant difference (p>0.05) in mucous glandular cells (
Table 4 and
Fig. 5). Nevertheless, the T-test analysis results for Ki67, denoted a significant increase (p<0.01) in epithelial, connective, and muscular tissues of affected esophagi compared to normal tissues while increased expression of this marker was not significant (p>0.05) in mucous glands (
Fig. 5B). The epithelial and muscular tissues of the affected esophagi showed a significantly increased number of TUNEL+ cells, which represented apoptosis, compared to the normal tissue samples (p<0.05). The number of TUNEL
+ cells according to manual cell counting results, was lower in the mucous glandular cells and connective tissue of the infected esophagi compared to healthy esophagi although the difference was not significant (p>0.05,
Table 4,
Fig 5). Likewise, the T-test analysis results for TUNEL assay exhibited a significant increase in apoptosis rate in epithelial and muscular tissues, although it was not significant in connective and mucous gland tissues between the groups (
Fig. 5B). It seemed that the rate of proliferation was higher than the rate of apoptosis in the nodules, except for the squamous epithelium of the esophagi affected with nodules, which showed a higher rate of apoptosis compared to proliferation (
Table 4).
 DISCUSSION
DISCUSSIONAccording to our findings, a total of 20 out of 152 dogs (13.6%) were diagnosed with esophageal nodules caused by spirocercosis during 2017- 2022. Moreover, the disease had the highest prevalence in the age group of 3-6-year-old dogs. Also, no neoplastic lesions were observed. Studies have reported a prevalence of 10-85% for canine spirocercosis, depending on the climate and urbanization of the area (
1). Moreover, we did not report any case of esophageal spirocercosis in dogs younger than 3 years old. This may be due to the occurance of esophageal nodules a few months after the primary infection, even dogs under six months of age are infected by spirocercosis without esophageal lesions and clinical signs (
1, 7). In addition, dogs of the present study were mostly kept as guard dogs in gardens or factories. According to studies, the prevalence of spirocercosis is higher in hunting dogs compared to house-kept pets as the chance of exposure to intermediate or paratenic hosts of the parasite is higher in dogs kept outdoors (
22). The present study reported mild anemia (HCT: 32-35%), neutrophilia, eosinophilia, and mild monocytosis in the infected dogs, which was compatible with the findings of previous studies (
25). The monocytosis observed in the present study can be explained by the fact that monocytes are precursors of tissue macrophages and nodular reactions (
26). Previous studies have reported the presence of anemia, neutrophilia, leukocytosis, and eosinophilia in dogs with spirocercosis (
27). Also, the IHC evaluations in the present study showed an abundant number of neutrophils, followed by T cells (CD3
+), B cells (CD20
+), and macrophages (CD68
+) in the nodules. These findings were in line with a study performing IHC investigation on lesions caused by
S. lupi in red foxes, reporting that CD3
+ T cells, CD68
+ macrophages, and CD79α
+ B cells were the most abundant inflammatory cells in the lesions in order of abundance (
28). Furthermore, similar to the results of Devir et al. (
10), the current research observed focal distribution of T cells and B cells in the periphery of the parasitic nodules. GDNF was significantly overexpressed in the nodular esophagi, while it showed no considerable expression in normal esophagi. Previous studies have reported the increased production of growth factors, including FGF, VEGF, and PDGF, in nodules and sarcomas caused by
S. lupi (
10). Although no study has ever reported the effectiveness of neurotrophic factors in canine spirocercosis, limited studies have investigated the role of GDNF in parasitic diseases. For example, a recent study reported the increased number of GDNF
+ macrophages in the intestines of rats infected with a tapeworm,
H. diminuta, suggesting the potential role of GDNF in response to intestinal infection. Thus, it is believed that GDNF has a neuroprotective, neurorestorative, smooth muscle-changing, and enteric neural system-plasticizing role in these kinds of infections (
11, 14). The present study reported the overexpression of GDNF in macrophages, fibroblasts, and muscle fibers present in nodules induced by
S. lupi. However, it was unclear whether these cells secreted GDNF or received the GDNF released from other sources. Moreover, it is believed that macrophages, fibroblasts, and muscle cells have GFRα1-4 receptors (
12), enabling them to express GDNF in response to some factors. However, as mentioned before, it seems that the normal esophageal tissue has no receptor for GDNF or the mature healthy cells in the canine esophagus are not affected by the mentioned factor. The same issue has been raised for FGF, VEGF, and PDGF (
10). Given the results of Devir and Clift (
5) and the nature of pre-neoplastic nodules with more collagen fibers and fibrocytes, overexpression of VEGF and FGF in the pre-neoplastic nodules more than early ones was reported. In this regard, our results indicated an overexpression of GDNF in fibroblasts and an overexpression of Ki67 in connective tissue of the parasitic nodules. Based on a study conducted by Devir and Clift (
5) all of our examined parasitic nodules were combined form. Hence, the nodules had a simultaneous increase in both collagen fibers and fibroblasts which may define the important role of the increment in connective tissue components in the pathogenesis of the disease.
On the other hand, we did not find a significant difference in p53 expression between nodular and normal esophageal tissues. As a tumor suppressor gene, p53 regulates the cell cycle and controls apoptosis in normal cells, while inducing apoptosis in malignant cells (
15, 16). Thus, its loss of activity can lead to the malignant transformation of normal cells. Moreover, the mutations of the p53 gene and abnormal accumulation of its protein have been reported in precancerous and cancerous conditions (
16). It is worth noting that molecular analyses can differentiate between wild-type and mutated p53 in case of p53 accumulation detected besides IHC staining methods (
29). In the present study, the TUNEL assay showed apoptosis in both normal and nodular esophageal tissues. However, the apoptosis rate was high in the inflammatory cells, especially neutrophils, and squamous epithelium of the infected tissue, while considerably lower in peripheral cells of the esophageal mucous glands and negligible in the muscular and connective tissues. It was significantly higher in the infected tissue compared to the normal tissue. In investigations wherein more pronounced cellular demise encompasses larger regions of tissue as opposed to isolated cells, it is frequently advantageous to quantify lesions based on the percentage of area impacted, whether that pertains to the entirety of the tissue or a designated tissue compartment (
24). In our research, given that we assessed various components of tissues (epithelium, connective, muscular, and mucous gland) and observed no significant discrepancies in severity across study cohorts, we conducted an analysis and comparison of apoptotic cell counts among different groups utilizing a quantitative statistical method (T-test).
Furthermore, the rate of proliferation, investigated using IHC staining for Ki67, was significantly higher in squamous epithelium, muscle cells, and connective tissue of the nodular lesions compared to normal tissue. Interestingly, the rate of apoptosis was up to 2-3 times higher in some areas of nodular esophagi than in the normal tissue. However, the proliferation rate was significantly higher than the apoptosis rate, about 6-8 times higher in the nodular tissue than in the normal tissue. Previous studies have reported similar activity of Ki67 in human and canine tissues. In normal skin, Ki67
+ cells are confined to the basal layer of the epithelium (
30). Furthermore, the application of Ki67 as a biomarker for detecting precancerous lesions has been approved in previous research. Thus, it can be used for early diagnosis of esophageal neoplastic lesions (
19). On the other hand, Ki67 has been used as a prognostic marker for several neoplastic lesions of domestic animals (
18). Hence, this marker can be applied as a predictive and prognostic biomarker in premalignant lesions that originate from both epithelial and mesenchymal tissues (
31, 32). Also, the TUNEL assay, which is commonly used for detecting DNA damage, is a powerful marker for apoptosis in biological systems, especially as a part of the complementary panel of biomarkers (
20).
CONCLUSIONAs a neurotrophic factor, GDNF plays an important role in the pathogenesis of nodular spirocercosis in dogs. Moreover, the rates of apoptosis and proliferation were significantly higher in nodular tissue, reaching 2-3 and 6-8 times higher than in the normal tissue in some areas, respectively. Finally, according to Ki67 expression results we concluded that the tissue components of nodular esophagi, including epithelium, connective tissue, and muscle fibers, may stimulate esophageal tissue to induce neoplastic transformations.
CONFLICT OF INTERESTThe authors declare that they have no known conflict of interest in the conduction of the current study.
ACKNOWLEDGMENTSThe authors would like to thank the Rasta Research Center for preparing the immunohistochemical sections.
AUTHORS’ CONTRIBUTIONSA and AA were involved in the conceptualization, investigation, methodology, software, writing of the original draft, reviewed and edited the paper. AA made the data curation, formal analysis, supervision, validation, and final approval of the paper.

 10.2478/macvetrev-2025-0014
10.2478/macvetrev-2025-0014








