Viral hemorrhagic septicemia (VHS), Infectious hematopoietic necrosis (IHN), and Koi herpesvirus disease (KHVD) are listed diseases by the European Commission that pose significant threats to the global aquaculture industry, resulting in substantial economic losses and impacting fish health and welfare. Due to their rapid spread potential, it is crucial for member states to implement measures preventing their transmission to disease-free areas. In this study, we aimed to assess the presence or absence of these viruses in fish aquaculture facilities in North Macedonia. During 9 years of surveillance from 2015 to 2023, 1,527 samples were tested for VHS and IHN, and 2,760 samples were tested for KHVD from aquaculture sites across North Macedonia using molecular diagnostic techniques. Our results indicated the absence of VHS and KHVD in all tested samples. However, the number of IHN-affected farms increased from two in 2018 to 33 by 2023, persisting across multiple sites. Despite the absence of VHS and KHVD, the ongoing presence and increasing incidence of IHN highlight the need to assess the effectiveness of existing biosecurity measures and disease management practices in the region. Ongoing surveillance and stringent biosecurity measures are essential for controlling IHN and preventing the introduction of other viral pathogens. Strengthening these measures is vital to ensure the long-term sustainability of the aquaculture industry in North Macedonia.
The aquaculture systems are designed in a way that enables infectious pathogens from the environment or wild fish to be transmitted to captive stocks. Most farm animal diseases originate from wild populations of closely related species (
1, 2). Stress can weaken the immune system of fish, making them more vulnerable to diseases. Farms can experience stress due to high stocking density and inadequate water quality. In aquaculture, viruses and bacteria can become more dangerous in terms of virulence. They can cause significant fish infections and are a global economic concern. Over the past 20 years, there has been significant progress in understanding the viruses that affect lower vertebrates, resulting in improved methods for detecting and managing various fish diseases. The World Organization for Animal Health (WOAH) publishes the International Aquatic Animal Health Code every year to improve the health of aquatic animals worldwide (
3). Reported diseases to the WOAH have a substantial impact on the international trade of aquatic animals and their goods.
Viral hemorrhagic septicemia virus (VHSV) belongs to the family Rhabdoviridae,
Novirhabdovirus genus, known for the high mortality rates in various fish species (
4). The virus’s ability to infect a wide range of hosts and its resilience in aquatic environments pose considerable challenges to fish farmers and fisheries managers. Approximately 80 species of freshwater and marine fish are affected by the VHSV (
5). Based on genetic variations, VHS has been classified into four main genotypes, referred to as Genotypes I-IV. These genotypes are categorized geographically and are associated with different strains of the virus (
6). VHS is mainly transmitted horizontally (
7). The optimal temperature for virus survival and multiplication is between 9 °C and 12 °C, and decreases at temperatures above 15 °C (
6). Young fish are more sensitive, but adult fish exposed for the first time can have high mortality rates. Rainbow trout weighing less than 3 g are most vulnerable to VHS, ranging from 5 to 90% mortality (
8).
The Infectious hematopoietic necrosis (IHN) virus, a member of the Rhabdoviridae family and
Novirhabdovirus genus, is recognized for causing significant mortality in various freshwater and anadromous fish species (
9). With a broad host range and the ability to persist in aquatic environments, this pathogen poses a substantial threat to aquaculture systems worldwide. Over 30 fish species, predominantly salmonids, are known to be susceptible to IHN virus infection (
5). Juvenile fish are particularly vulnerable to IHN, with mortality rates ranging from 50 to 90% during outbreaks. Stress factors such as overcrowding, poor water quality, and abrupt environmental changes exacerbate the severity of the disease. Horizontal transmission through direct contact with contaminated water, infected fish, and contaminated equipment is the primary mode of spread. The replication of the IHN virus is optimal at water temperatures between 10 °C and 15 °C, making outbreaks more prevalent during spring and early summer when water temperatures align with this range (
9).
Koi herpesvirus disease (KHVD) is a very contagious disease of carp that has spread globally during the last 20 years. The disease is caused by Cyprinid herpesvirus-3 (CyHV-3) and is a pathogen for the common carp (
Cyprinus carpio carpio), ghost carp (
Cyprinus carpio goi), koi carp (
Cyprinus carpio koi), and common carp hybrids (
5). Koi herpesvirus is a member of the
Cyprinivirus genus in the family Alloherpesviridae. Molecular evaluation confirmed minor variations among KHV isolates in outbreaks from different parts of the world (
10). During outbreaks in common carp, the mortality rate ranges between 50% and 95% (
11, 12). Clinical outbreaks with mortality are usually detected in summer when the water temperature is between 15 ºC and 28 ºC. They are most commonly occurring in areas with high density and/or poor water quality. This disease is related to stress and may occur during high temperature alterations of the water or transportation (
13, 14). It is critical to recognize that infected fish may remain in a latent or carrier state for extended periods and become lifelong carriers with no clinical indications (
15).
Fish diseases of major importance in North Macedonia’s aquaculture and which are part of the Annual order for animal health protection for aquatic animals include VHS, IHN, infectious pancreatic necrosis (IPN), Spring viremia of carp (SVC), and KHVD. These diseases have devastating effects on aquaculture and are notifiable in many countries, including North Macedonia. The Food and Veterinary Agency is the authority responsible for the implementation of surveillance programs for viral diseases in North Macedonia. Regular monitoring for notifiable fish diseases in North Macedonia was initiated in 2015 as part of the country’s efforts to align with European Union requirements and fulfill the criteria for specific negotiation chapters. Before 2015, no systematic data or records regarding the presence of these diseases were available. During the surveillance program conducted between 2015 and 2018, no cases of VHS or KHVD were detected. However, in 2018, IHN was reported for the first time in the country, as part of this monitoring program. The case was documented by Cvetkovikj et al. (
16).
Based on the data collected up to 2019, it was hypothesized that the incidence of VHS, KHVD, and IHN in the fish farms of North Macedonia may increase after 2019, particularly if biosecurity measures are not strengthened. The study aimed to investigate the occurrence of VHS, IHN, and KHVD in aquaculture facilities across North Macedonia.
MATERIAL AND METHODSFish were handled and sampled according to standardized methods described in the Commission Implementing Decision (EU) 2015/1554 of 11 September 2015 (
17) which align with national legislation. Written consent for the use of the data was obtained from the Food and Veterinary Agency, in compliance with regulatory requirements (Consent No. 02-232/2 from 4.11.2022).
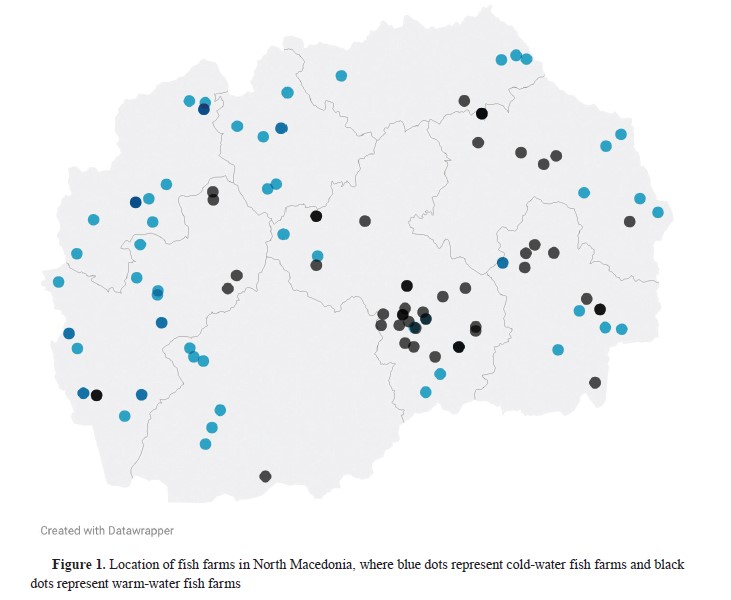
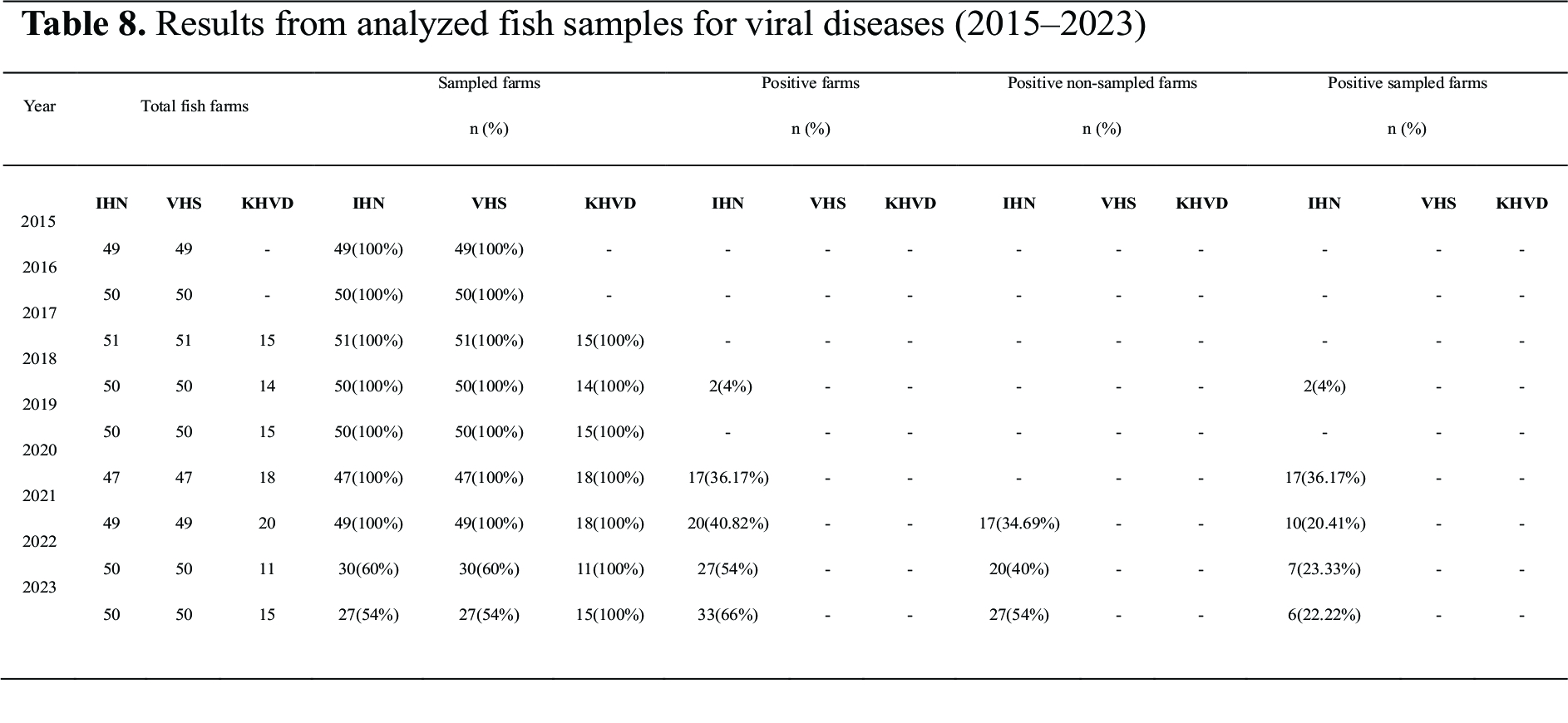
Samples were collected from fish farms located in different regions of North Macedonia as part of the national surveillance program conducted between 2015 and 2023. The study was conducted from 2020 to 2023, with additional data from the previous years used for comparison. The samples were collected only from registered fish farms by authorized veterinarians. If a single sample from a fish farm was tested positive, the entire farm was declared to be positive on the disease. During this period, 57 trout fish farms and 43 carp fish farms were registered and were operating (
Fig. 1). The number of farms fluctuated from year to year, and not all had fish year-round. Monitoring for VHS and IHN for the years 2015 and 2016 was conducted twice a year, once in spring and autumn. From 2017 to 2023, monitoring for VHS and IHN was conducted in autumn. From 2020 to 2021, samples were collected from all fish farms across the country for IHN and VHS. However, starting in 2022, samples were only taken from farms previously confirmed as negative for IHN and VHS. The remaining farms were considered positive for IHN and were excluded from sampling for both diseases. Additionally, the number of samples was reduced due to the adaptation of the program by the Food and Veterinary Agency.
Rainbow trout (
Oncorhynchus mykiss) was sampled for VHS and IHN. The sampling was conducted when water temperatures ranged from 8 to 15 °C. From each site, three pooled samples were collected, with each pool consisting of organs (heart, spleen, kidneys) from 10 fish.
Samples for KHVD were collected from various ponds on each carp fish farm during the warmer months when water temperatures ranged between 20 and 28 °C, twice a year, in late summer and early autumn. The number of sampled fish farms varied greatly from year to year because not all farms had fish year-round. Some farms were used only for stocking, and once the fish reached a certain weight, they were transferred to other farms.
In 2022 and 2023, sampling was only conducted in early autumn. A total of 15 samples were collected per fish farm, each sample comprised two
C. carpio fish (n=2). Organ pools were created using gills and head kidneys collected from two fish. Samples from fish were collected in minimum essential medium and then transported to the laboratory under cooling conditions for further analysis.
From 2015 to 2019, the samples were tested at the Institute of Veterinary Medicine of Serbia (NRL for fish disease), where VHS and IHN diagnostic was done on the recommended Bluegill fry-2 (BF-2) and Epithelioma papulosum cyprini (EPC) cell lines based on the recommendations by the European Commission (
17). In addition to testing for viruses in the cell cultures, samples were also analyzed for VHS and IHN using RT-qPCR according to the protocols described by the European Commission (
17). After 2020, the samples were analyzed at the Faculty of Veterinary Medicine - Skopje by using only RT-qPCR method according to the recommended protocols of the European Commission (
17). Both institutions used only molecular diagnostic techniques for detecting KHV in all samples as previously described (
18).
The data was further analyzed by grouping fish farms according to administrative divisions. This analysis reported the total number of fish farms, the number of sampled fish farms out of the total, the number of positive fish farms, positive non-sampled fish farms, and positive sampled fish farms for each year from 2020 to 2023. Analysis of earlier years was not feasible as the samples were collected without prior knowledge of their source (blind samples).
Fish farms that were depopulated either by the authorities or by the owners were included in the sampling process.
RNA extractionRNA was extracted using the SaMag 24 automated extraction system by Sacace Biotechnologies in conjunction with the SaMag Total RNA/DNA extraction kit following the standard protocol provided by the manufacturer. Initially, 25 mg of aggregates were homogenized in a stainless-steel Eppendorf tube along with a metal ball using the MM Mixer Mill 400 (Retsch) for 5 min at 22 Hz/min. Subsequently, the homogenized mixture was briefly centrifuged. Following the initial sample processing, 200 μl of supernatant from all samples underwent nucleic acid extraction. In order to ensure optimal RNA for the subsequent PCR reaction, both the quantity and purity of the extracted RNA were assessed using a UV spectrophotometer (NanoDrop 2000C, Thermo Scientific), with the determination of the A260/280 ratio.
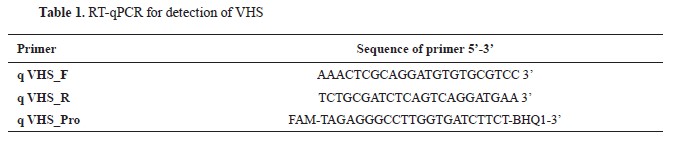
Virus detectionFor the detection of VHS, the RT-qPCR protocol (
Table 1) was used, designed by Jonstrup et al. (
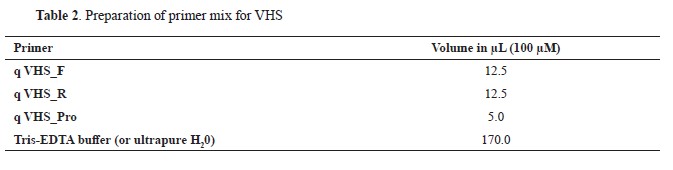
19). The RT-qPCR reaction was conducted using the QuantStudio 5 system manufactured by Applied Biosystems, coupled with the Path-ID™ Multiplex One-Step RT-PCR Kit provided by Ambion (Applied Biosystems). Primers designed for this study targeted a specific sequence within the VHS virus genome. The composition of the reaction mixture in a total volume of 22.50 μL was as follows: 14.25 μL of DEPC treated water, 6.25 μL of 4x TaqMan Fast Virus 1-Step Master Mix, and 2 μL of the primer mix specific for VHS (
Table 2). To ensure reliability and reproducibility, a standard curve approach was implemented. Calibration of the assay was conducted using reference material designated as VHS Standard, with a predefined Ct (threshold cycle) value. The thermal cycling protocol for the RT-qPCR reaction was optimized as follows: an initial reverse transcription step at 50 °C for 5 min, followed by a denaturation step at 95 °C for 10 min. Subsequently, 40 cycles of amplification were performed, consisting of denaturation at 95 °C for 5 sec and annealing/extension at 60 °C for 30 sec. This stringent protocol ensured reliable and sensitive detection of the VHS virus in the analyzed samples.
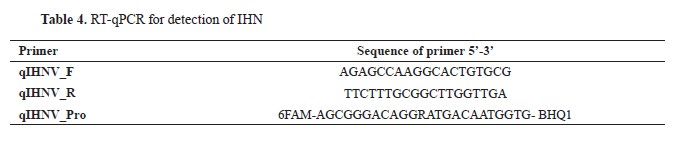
For the detection of IHN, two RT-qPCR protocols have been employed over time. Until May 2021, the RT-qPCR protocol designed by Purcell et al. (
20) was the standard (
Table 3). However, after May 2021, the protocol developed by Cuenca et al. (
21) became the primary method (
Table 4). The primers used in this study amplified a short sequence in the N gene. The nucleotide sequences of the primers, as well as the method of preparation of the primer mixtures, are given in Tables 3, 4, and 5. The reaction was carried out in a total volume of 22.50 μL, with the following composition of the mixture: 14.25 μL DEPC treated water, 6.25 μL 4x TaqMan Fast Virus 1-Step Master Mix, and 2 μL primer mix for IHN (
Table 5). The thermal cycling protocol consisted of an initial incubation at 50 °C for 2 min, followed by denaturation at 95 °C for 10 min. This was succeeded by 40 cycles of amplification, each comprising 15 sec at 95 °C and 1 min at 60 °C.
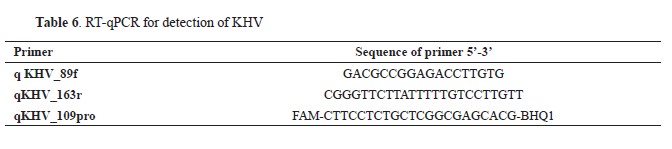
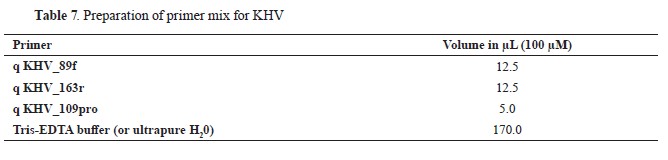
For the detection of KHV in carp samples, a real-time quantitative PCR (qPCR) assay was conducted utilizing primers and a probe designed by Gilad et al. (18) (
Table 6). The RT-qPCR reaction was conducted using the QuantStudio 5 system manufactured by Applied Biosystems, coupled with the Path-ID™ Multiplex One-Step RT-PCR Kit provided by Ambion (Applied Biosystems). The thermal cycling protocol for the RT-qPCR reaction was optimized to ensure accuracy and sensitivity. It commenced with an initial reverse transcription step set at 50 °C for 5 min, followed by a denaturation step at 95 °C for 10 min. Subsequently, 40 cycles of amplification were meticulously performed, consisting of denaturation at 95 °C for 5 sec and annealing/extension at 60 °C for 30 sec (
Table 7).


 RESULTS
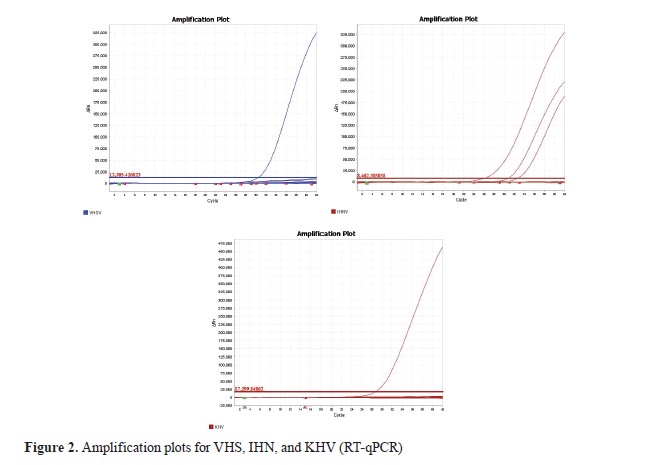
RESULTSThe results showed no detection of VHS or KHVD in fish farms in North Macedonia (
Fig. 2). IHN was initially detected in 2018 at two fish farms, which were subsequently depopulated to mitigate the outbreak. By 2020, the number of IHN-positive farms rose significantly to 18. However, one of these farms was depopulated and its status was subsequently marked as unknown. In 2021, IHN was detected at 10 fish farms, three of which were newly infected. The total number of IHN-positive fish farms was 20.
In 2022, samples were collected from 30 fish farms, representing 60% of all active farms. Seven additional farms tested positive for IHN, increasing the cumulative total to 27. In 2023, sampling was conducted at 27 fish farms, accounting for 54% of the total. Six additional farms tested positive for IHN, resulting in a cumulative total of 33 IHN-positive fish farms (
Fig. 3,
Table 8).
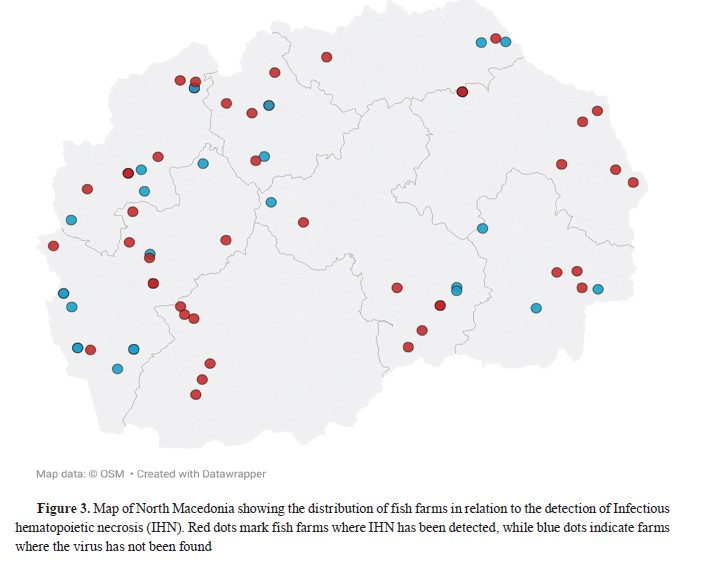
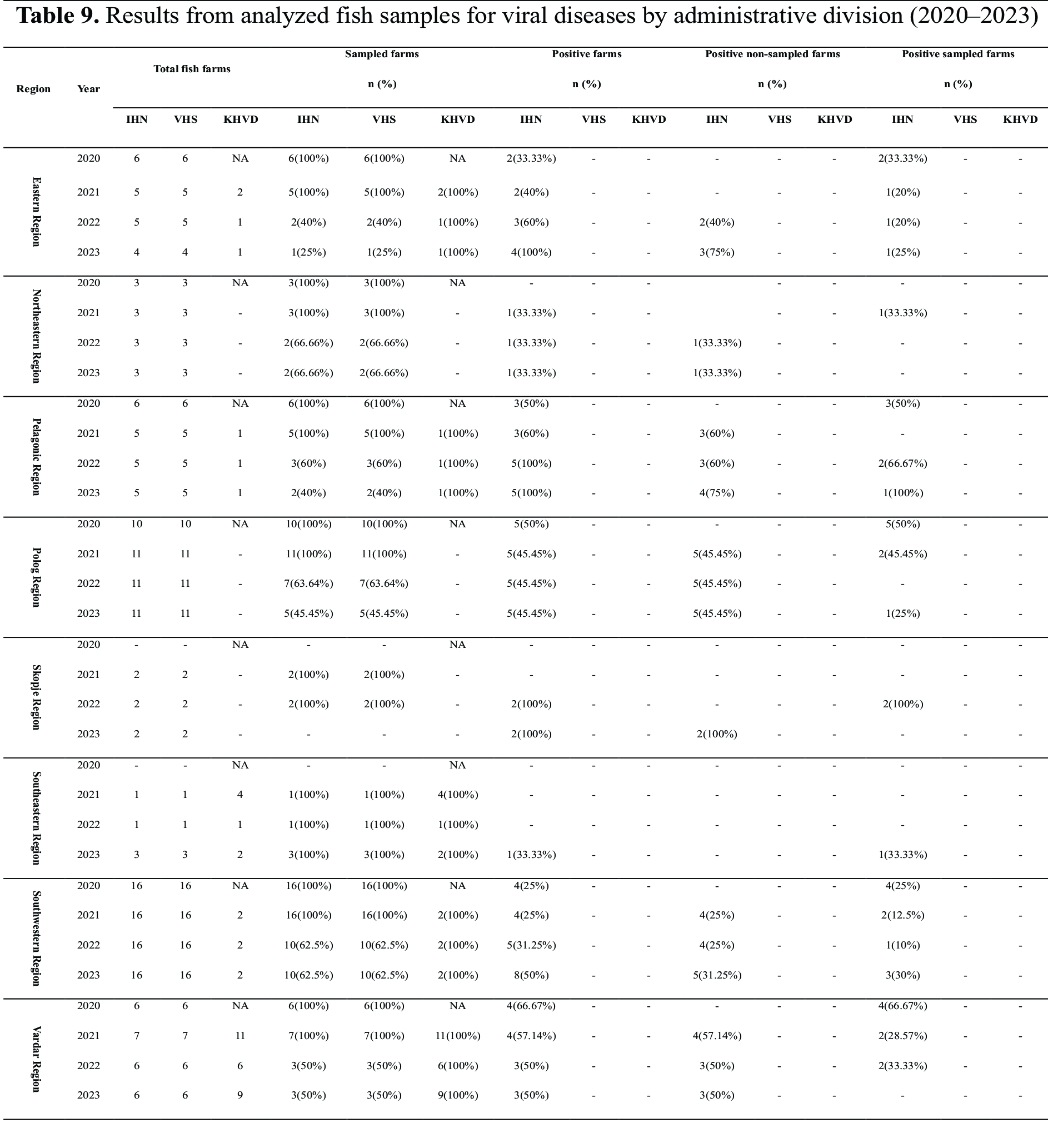
Eastern Region - In 2020, positive samples were identified from two fish farms, both of which were officially declared positive. In 2021, one positive fish farm was detected, but no new fish farms were declared positive. In 2022, one fish farm was identified as newly infected. In 2023, one additional fish farm was newly infected.
Northeastern Region - In Northeastern Region IHN was first detected in 2021, with only one positive fish farm identified. No additional infections were recorded in this region throughout the study period.
Pelagonic Region - In 2020, positive samples were identified from three fish farms, all officially declared positive. In 2021, all samples tested negative. However, in 2022, two new fish farms were declared positive. In 2023, one additional fish farm was reported as infected.
The Polog Region experienced the highest number of positive fish farms in 2020. Five fish farms were declared positive. In 2021, no new fish farms were reported as infected. In 2023, there were positive samples from existing positive fish farms.
In the Skopje Region, IHN was first detected in 2022. All samples collected were positive, and the infections were traced to 2 fish farms.
In the Southeastern Region, IHN was detected for the first time in 2023, with only 1 fish farm tested positive.
In the Southwestern Region, 4 fish farms were declared positive in 2020. In 2021, 4 fish farms tested positive, with no new fish farms reported as infected. In 2022, 1 new fish farm was declared positive. In 2023, 3 new fish farms were reported as infected.
In the Vardar Region, 4 fish farms were declared positive in 2020. In 2021, 2 additional fish farms were reported as infected. In 2022, 1 more fish farm was declared positive. However, in 2023, although samples were collected, all tested negative.
Detailed data are presented in
Table 9.
DISCUSSIONThis study showed that VHS and KHVD were not present in the aquaculture facilities in North Macedonia up to 2023. The PCR setup was validated, confirming optimal primer performance and reaction conditions. Nevertheless, there were no amplification signals found in any of the samples tested that would have shown the presence of VHS and KHVD, i.e. all samples tested negative (
Fig. 2). This result is significant because these two viral diseases are known to be highly contagious and cause high mortality in farmed fish.
Throughout Western Europe’s history, freshwater salmonids have been closely associated with VHS, which has been widely reported in aquaculture, particularly in Germany, Italy, France, and Denmark (
22). In the Balkan region, cases have been documented in Bulgaria (
23), Croatia (
24), and Slovenia (
25).
The first reports of KHVD outbreaks were from farms with high fish density from Israel (
26), Germany (
27), and the USA (
28). It was later identified in Austria, Belgium, Denmark, England, Wales, France, Italy, Luxemburg, Netherlands, Switzerland, Sweden, Poland (
29), Czech Republic (
11), Romania (
30), Slovenia (
31), Croatia (
32), different parts of Asia (
33), and in Canada (
34).
The absence of VHS and KHVD is an encouraging indicator that existing biosecurity and disease management strategies may be effective in mitigating their introduction and spread.
Conversely, the study highlighted a concerning trend with IHN outbreaks. The disease was first identified in the 1950s in sockeye salmon (
Oncorhynchus nerka) within American hatcheries, where initial outbreaks resulted in severe mortality rates among juvenile fish. Since then, IHN has been reported in North America, Europe, and Asia, particularly in regions with intensive aquaculture operations (
9).
The first European outbreaks were documented in France during the 1980s, with subsequent reports from Germany and Italy (
35). In the Balkans, outbreaks have been reported in Slovenia (
36), Croatia (
37), Kosovo (
38), and North Macedonia (
16).
After it was first reported in 2018 by Cvetkovikj et al. (
16), the disease steadily increased in incidence with a significant spike in 2020 (
39). A concerning trend observed in several regions was the detection of newly infected fish farms across the years. In Eastern, Pelagonia, and Southwestern regions of North Macedonia, newly infected fish farms were detected indicating the continued risk of IHN transmission between farms. The findings suggest that IHN was likely circulating even in 2019, but it went undetected at that time. Encouraged by the seemingly favorable results from 2019, fish farm operators mistakenly believed the issue had been resolved. As a result, they resumed normal operations without implementing the necessary precautions, assuming the risk had passed. This complacency created an opportunity for IHN to spread unnoticed, leading to its transmission to additional fish farms.
Moreover, there is a significant lack of understanding regarding the presence of IHN in natural water bodies such as rivers. This gap in knowledge about the potential impact of the virus on native fish populations in North Macedonia further complicates efforts to control and prevent the disease. Without comprehensive surveillance and awareness, the full extent of the virus’s impact on both farmed and wild fish remains unclear, highlighting the need for increased research.
One of the reasons why KHVD and VHS were not detected during this period is the widespread effects of IHN, which overshadowed these other viral diseases. This trend has raised significant concerns among fish farmers regarding the increasing risks of viral infections. The significance of measures and monitoring to reduce the risks of viral infections in aquaculture has become evident due to the introduction and effects on fish populations.
Global trade and transportation of live fish continue to present challenges for biosecurity measures, as proven by reported viral disease outbreaks in aquaculture facilities across the globe (
6, 9, 40). Therefore, it is crucial to uphold strict biosecurity measures such as quarantine for imported fish, regular health checks, and disinfection protocols to reduce the chance of diseases being introduced and spread.
North Macedonia’s geographical characteristics, such as its separation from other bodies of water and a small number of migratory fish, might have also decreased the chances of viral introduction.
Although the surveillance findings may indicate a reduced current threat of VHS and KHVD outbreaks in North Macedonia, it is crucial to take proactive steps to maintain alertness and readiness. Additionally, the possible effects of environmental factors on the dynamics of viral diseases must not be disregarded. Variations in water temperature, pollution levels, and habitat degradation can impact the vulnerability of fish populations to viral infections and potentially exacerbate disease outbreaks in specific situations (
6, 9, 40). Implementing sustainable aquaculture practices is essential for reducing disease risks and ensuring the ongoing success of aquaculture operations. Staying alert is crucial to prevent the possible appearance of new diseases or the re-emergence of familiar viruses via different routes. Climate change, habitat destruction, and human activities can lead to the development and transmission of infectious diseases. Thus, it is crucial to have continuous monitoring, collaboration, and cooperation among researchers, fish farmers, and regulatory agencies to effectively manage and prevent diseases.
In general, this program provides important knowledge about the occurrence and control of major fish diseases in North Macedonia, emphasizing the significance of surveillance and preventive actions in fish farming practices. Understanding the health status of fish is essential for implementing sustainable management practices.
CONCLUSIONThis study confirms the absence of VHS and KHVD in aquaculture facilities in North Macedonia while reporting the emergence of IHN in previously disease-free farms. These findings highlight the critical role of stringent biosecurity measures and effective disease management strategies in preventing the introduction and spread of viral pathogens in aquaculture systems. Continuous surveillance, early detection, and collaboration among fish farmers and veterinary authorities remain essential for maintaining the health and sustainability of the aquaculture sector in the region. Further research should focus on refining diagnostic techniques, understanding transmission dynamics, and developing targeted prevention strategies to mitigate the risks associated with emerging viral diseases in aquaculture.
CONFLICT OF INTERESTThe authors declare that they have no known conflict of interest in the conduction of the current study.
ACKNOWLEDGMENTSThe authors would like to acknowledge the Faculty of Veterinary Medicine - Skopje, Ss. Cyril and Methodius University in Skopje and the Food and Veterinary agency of North Macedonia for sharing their logistical, intellectual, and research infrastructure for this study.
AUTHORS’ CONTRIBUTIONAT conceptualized the study, collected and analyzed the samples and wrote the manuscript. ID and KK supervised the sample analysis. ZP carried out the sample analysis. MN was included in the hypothesis formulation and manuscript writing. AG was coordinating fieldwork. AC supervised the study and manuscript writing. All authors have revised and approved the final version of the manuscript.

 10.2478/macvetrev-2025-0015
10.2478/macvetrev-2025-0015











