This study aimed to formulate, characterize, and investigate the effects of herbal extract emulsions (HEE) on the growth of broiler chickens. The HEE were prepared with 0.3% herbal extracts, pharmaceutical excipients, and a 5% brown sugar solution as the continuous phase. The study was conducted with five groups, each with four replications, and three chicks per replication (completely randomized design). Mixed-sex broilers (n=60) were treated with 0.03% HEE in drinking water for five weeks, whereas one control group (n=12) received only regular drinking water. The preference rate for HEE in drinking water was determined by observing the consumption quantity over a designated period. Broiler chicken growth, including live weight, internal organ weight, carcass characteristics, percentage of abdominal fat, and feed conversion ratio (FCR) was observed in 40 birds slaughtered at the end of the trial. The 0.3% HEE preparations comprising moringa leaf extract, turmeric rhizome, or a mixture of both, were characterized as oil-in-water (o/w) emulsions, with a pH range of 5.6-6.5, and viscosity of 0.05-0.06 Pa.s. The treatment groups had significantly higher preference for the HEE-supplemented drinking water compared to the control. The mean live weight was 1,721.67-1,744.17 g/bird, mean internal organ weight 220.00-253.33 g/bird, carcass percent 70.41-74.20%, abdominal fat percent 0.90-1.94%, and mean FCR 1.55-1.60. This study showed that the HEE preparations were preferred and significantly influenced internal organ and abdominal fat weight. The carcass weight and FCR were non-significantly unaffected by HEE.
The utilization of herbs in broiler feeds has been extensively examined within the scientific community. This includes incorporating turmeric rhizome powder into the diet, which demonstrated a considerable impact on broilers’ protein digestibility and growth performance (
1, 2). The administration of moringa leaf flour at a dosage range of 200–600 g/100 kg of feed has been an adequate substitute for oxytetracycline in promoting growth in broiler chickens (
3, 4). Similarly, incorporating a combination of herbal powders, comprising turmeric, ginger, and basil, has also been shown to possess comparable growth-promoting properties (
5). Additionally, using a blend of traditional ingredients derived from multiple rhizomes, including turmeric, ginger, lemongrass, and Curcuma, has also been reported to elicit similar effects (
6).
Nevertheless, several challenges remain. The combination of herbs is not optimal, and the administration of herbs in the form of flour mixed into the ration does not ensure the appropriate dose consumed by livestock. Moreover, there is nonhomogeneous mixing of feeds, and the consumed dose by livestock is not uniform. Furthermore, the administration of extracts through drinking water has not reached an appropriate concentration. In some cases, it may even reduce the amount of drinking water chickens consume due to the bitter taste of certain extracts. Consequently, the efficacy of the herbal feed in terms of conversion and efficiency, has not reached the optimal level of bioavailability, which in turn affects the growth of the chickens. Therefore, it is necessary to design and develop an easy preparation for chicken that would ensure appropriate dose with maximum consumption and bioavailability.
The turmeric rhizome (
Curcuma longa L.) contains a curcumin compound, which is helpful as an anti-oxidant and anti-microbial agent. It has positive effects on the immune system (
7) and improves the function of the digestive organs by stimulating bile and pancreatic secretion which contains amylase, lipase, and protease enzymes. These enzymes can improve the digestion of feed ingredients such as carbohydrates, fats, and proteins (
1, 8). Moringa leaves (
Moringa oleifera) are rich in vitamins, minerals, amino acids, alkaloids, flavonoids, and phenolic compounds such as zeatin, quercetin, kaempferol, apigenin, and various other phytoconstituents (
9, 10). These compounds are beneficial as natural alternative antibiotics, improve immune responses (
11), maintain cecal microbial structure, and are used as growth promoters improving the performance of broiler chickens (
12, 13). This study aimed to formulate and characterize the emulsion dosage form of
Moringa oleifera Lam extract and turmeric (
Curcuma longa L.) extract and investigate its effect on the growth of broiler chickens.
MATERIAL AND METHODSPreparation of herbal extractsTurmeric rhizomes and moringa leaves were obtained from Medan Central Market in Indonesia. The plants were processed into turmeric and moringa leaf extracts in phytochemistry laboratory, Faculty of Pharmacy, University of Sumatera Utara. Turmeric powder and moringa leaf powder were macerated in ethanol 96% for eight days. After that, both macerates were evaporated with a rotary evaporator at 50 °C and 100 mbar to obtain concentrated turmeric extract and moringa leaf extract.
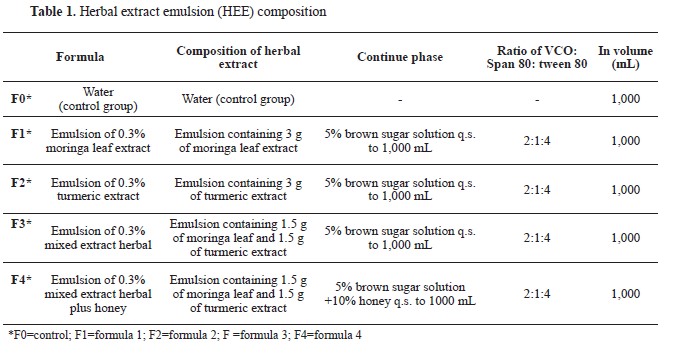
Formulation of the 0.3 % herbal extract emulsions (HEE)The 0.3% herbal extract emulsions (HEE) were prepared by emulsification methods involving mixing turmeric, moringa leaf, and a combination of both extracts with pharmaceutical excipients in varying ratios. The formulations of the 0.3% HEE are presented in
Table 1 for reference. The following excipients were utilized: virgin coconut oil (VCO) as the oil phase (Javara), span 80 (Merck), and tween 80 (Merck) as the emulsifying agent. The continuous phase consisted of a 5% solution of brown sugar obtained from the local central market. The herbal extracts, VCO, Tween 80, and Span 80 were homogenized in a glass beaker using a magnetic stirrer (MS-H280-PRO) at 500 rpm for 15 to 30 min. These liquid mixtures were designated as the oil phases. Subsequently, the aqueous phases (5% solution of brown sugar) were gradually introduced to the 1,000 mL volume and mixed with a magnetic stirrer at 500 rpm until a uniform emulsion was achieved (approximately 30–60 min).
 Physical characteristics of the HEE
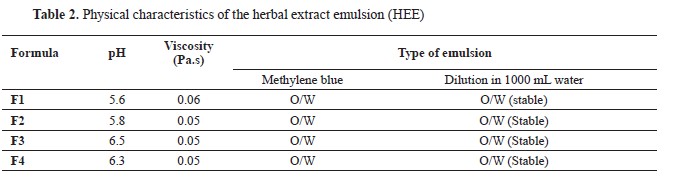
Physical characteristics of the HEEThe physical characterization tests of the 0.3% HEE formulas were conducted to determine the pH of HEE using a pH meter (
Bench Top 86502), the viscosity by using a viscometer (NDJ-85), and the emulsion type determined by the methylene blue method. The HEE were diluted in 1,000 mL of water. The results of these tests are presented in
Table 2.
The water dilution method demonstrated the stability of the HEE preparations, as no breakage or precipitation occurred. This characteristic was expected, given that the supplementation of herbal extracts through drinking water is achieved with the correct dose and an easily diluted preparation. This process involved the preparation of an emulsion containing 0.3% herbal extracts at a specific dose. This emulsion was then diluted in 1,000 mL of water to obtain the desired emulsion concentration containing 0.03% herbal extracts, which was administered as drinking water to the broiler chickens daily.
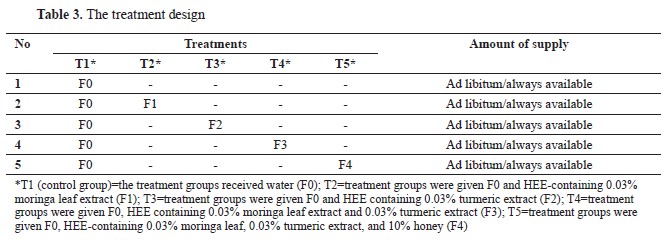
Treatment designThe study was conducted using a completely randomized design (CRD) with five treatments and four replications (5×4×3). Sixty mixed-sex CP-707 broilers were used in the present study. The chickens were divided into five groups, each consisting of four subgroups, with three chicks per subgroup. Broilers were acquired from the PT Charoen Pokphand Indonesia, Tbk. The broiler breeding process lasted for 35 days and involved the use of three different commercial feed products from PT Charoen Pokphand Indonesia Tbk. The broilers were offered a diet consisting of 311 B pre-starter feed for the initial seven days, 511 B starter feed for the subsequent 18 days, and 512 B finisher feed for the final ten days. Moreover, four treatment groups were administered two different beverages daily for 35 days: 1) emulsion containing 0.03% herbal extract and 2) regular drinking water. In contrast, one control group received only regular drinking water. Both drinks were provided in unlimited amounts or with constant availability. The broiler’s feed intake was determined by subtracting the quantity of raw feed provided from the quantity of remaining feed each day. An analogous approach was employed to determine the volume of potable water. In order to determine the weight gain, the original weight of each grill was documented and thereafter monitored on a weekly basis. The 0.03% emulsion preparation was prepared by adding 1 of the emulsion preparations containing 0.3% herbal extract, then diluted in 10 L with 5% brown sugar solution in order to obtain an emulsion preparation that had a concentration of 0.03%. The treatment design is shown in
Table 3.
 Evaluation of treatments
Evaluation of treatmentsThe preference rate of formula HEE on drinking water consumption was observed in the consumption of drinking water of broiler chickens in five weeks. The effect of the treatment on the final weight, carcass weight, internal organ weight, and abdominal fat weight of the chickens was determined in 40 birds slaughtered at the terminal stage of the trial. The percentage of carcasses was calculated based on the carcass weight to live weight ratio. The carcass weight was obtained by subtracting the weights of the head, feet, internal organs, abdominal fat, blood, and neck. The percentage of abdominal fat in broilers refers to the amount of fat accumulatings in the abdominal cavity in comparison to the bird’s total body weight.
Ethical statementAll experimental animals and protocols in this study were approved by the animal ethics committee Universitas Sumatera Utara, with approval number 0243/KEPH-FMIPA/2019.
Statistical analysisThe parameters were analyzed in IBM® SPSS® Statistics version 22 software. The initial analysis entailed investigating the assumptions that must be met in performing a one-way ANOVA analysis, which included the normality test and the homogeneity of variances test. The assumption of normality was evaluated by the Kolmogorov-Smirnov test, and this can also be observed by examination of the histogram, boxplot, and stem-and-leaf plot, all of which were included as output from the Kolmogorov-Smirnov test. Meanwhile, the assumption of a homogeneous variance was assessed by applying Levene’s test within a series of one-way ANOVA tests, which is included in the analysis results output. The results of the analysis demonstrated that all parameters were normal and homogeneous, with the exception of the impact of treatment on the percentage of abdominal fat. Subsequently, a one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was conducted. Additionally, the Kruskal-Wallis test was employed to examine the abdominal fat parameter. The significance level was set at 0.05, and all measurements are presented as the mean ± SD.
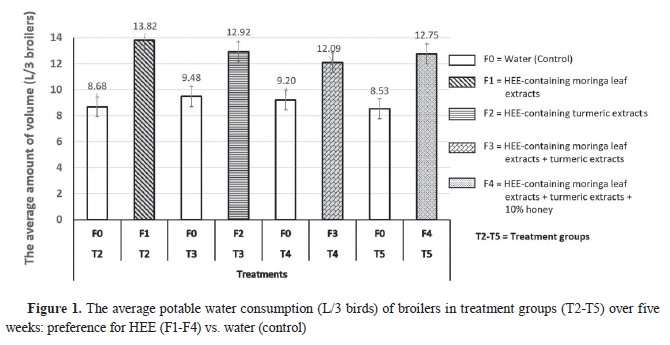
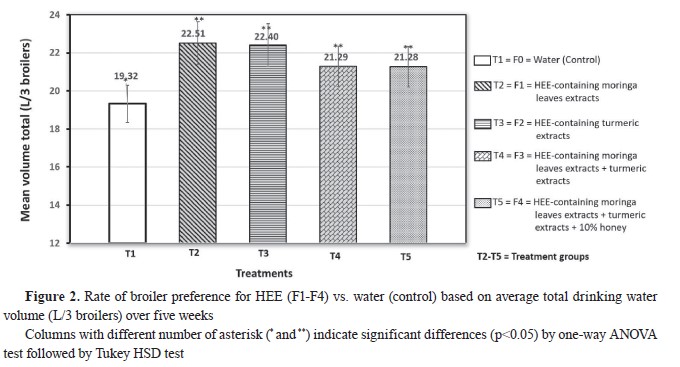
RESULTSPreference level of broiler chickens on HEE (F2-F4) and water (control) as drinking waterThe preference level of broiler chickens for HEE preparations and control over a five-week period is illustrated in
Fig. 1 and
2. Тhe consumed quantity of HEE preparations in the treatment groups (T2-T5) was significantly higher than the control (T1). The consumption of HEE in T2 and T3 was nonsignificantly higher than the other treatment groups.
Effect of treatment on live weight of broiler chickensThe live weights among the treatment groups were non-significantly different, ranging from 1,721.67 g to 1,744.17 g.
Effect of treatment on internal organs of broiler chickens
The total weight of internal organs had significant variation among the treatment groups. T4 had significantly higher and T5 had significantly lower total organ weight compared to the other groups. Non-significant differences were observed between T1, T2, and T3.
The liver weights showed statistically significant differences, ranging from 34.08 g in T5 to 43.80 g in T3. T3 had significantly higher liver weight compared to T2, T4, and T5, but not with T1. T1 had significantly higher liver weight compared to T2 and T5. T2 and T5 had significantly different liver weights among them and compared to the other groups.
T3 had significantly higher (11.24 g) and T2 significantly lower (6.49 g) heart weight compared to the other groups as well as among them. T4 and T5 were non-significantly different among them, but differed significantly compared to the other groups. T1 alone, had significantly different heart weight compared to the other groups.
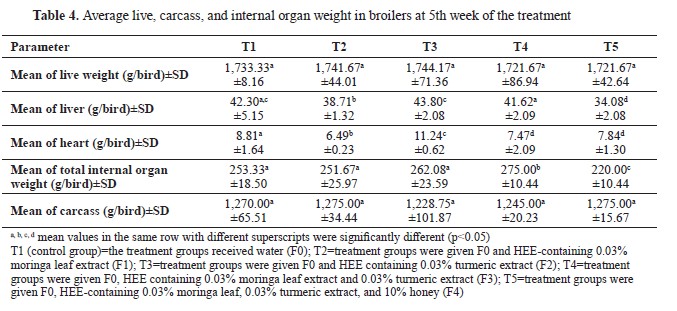
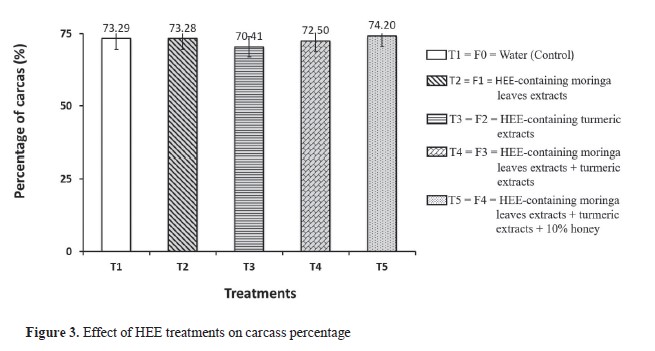
Influence of treatment on carcass percentageThere were no significant differences in the carcass weight among the groups (
Table 4). T1, T2, and T5 had similar values ranging between 1,270.00 and 1,275.00 g. T3 and T4 had an average carcass weights of 1,228.75 g and 1,245.00 g, respectively. The carcass percentage ranged from 70.41% (T3) to 74.20% (T5) (
Fig. 3).
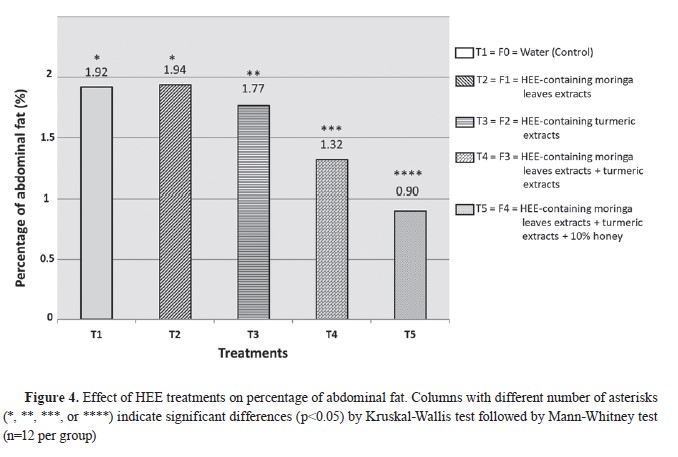
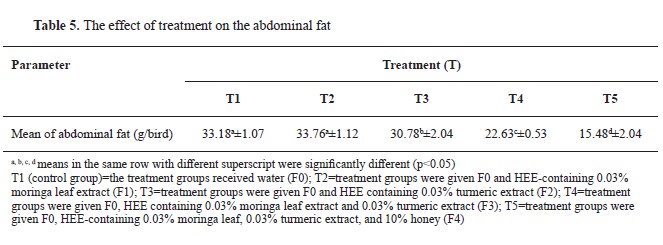
Effect of treatment on the percentage of abdominal fat
The percentage of abdominal fat ranged from 0.90% to 1.94%. T1 and T2 had non-significant differences. The mean abdominal fat ranged from 15.48 (T5) to 33.76 g/bird (T2) The groups T3, T4, and T5 had significantly lower percentage and mean value for abdominal fat compared to the first two groups. T4 has significantly lower value than T3, and T5 had significantly lower value compared to all other groups (
Fig. 4,
Table 5).
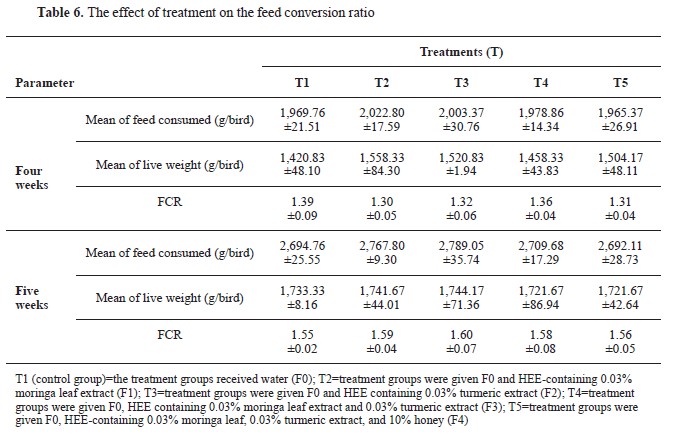
Effect of treatment on the feed conversion ratio (FCR)
Table 6 displays the treatment’s influence on the feed conversion value, which is also referred to as the feed conversion ratio (FCR). After analyzing the four-week data, it was discovered that the mean feed consumption did not vary significantly between the treatments. T2 had the highest mean (2,022.80 g/bird), while T5 had the lowest (1,965.37 g/bird). The mean live weight recorded in T2 was 1,558.33 g/bird, while the lowest was 1,420.83 g/bird in T1. Around 1.30 to 1.39 was the FCR value at week 4. T2 had the highest FCR of 1.30. T2 and T3 demonstrated a slight increase in feed consumption, with T3 exhibiting the highest intake (2,789.05 g/bird). The live weights of T2 and T3 were marginally higher. The FCR values for all treatments increased between 1.55 and 1.60, as anticipated, as the birds aged. T3 exhibited the highest FCR (1.60), indicating a slight decrease in feed conversion efficacy.
The results of the analysis of diversity with one-way ANOVA showed no significant difference between the control treatment groups (T1), T2, T3, T4, and T5 at week 4 with probability (p>0.05), [F(4.15)=1.578, P=0.23] and the same for the fifth week with a probability of 0.76 [F(4.15)=0.457, P=0.766].
 DISCUSSION
DISCUSSIONThe current study demonstrated that the administration of the HEE preparations containing moringa leaf extract, turmeric rhizome, or a combination of both extracts significantly increased the amount of drinking water consumed by broiler chicks (p<0.05). The consumption patterns of the emulsions in the treatment groups differed significantly from those of the control group, which was administered with ordinary water (p<0.05). This revealed that broiler chicks preferred water containing herbal extracts, potentially opening up new options for improving poultry health and farming techniques. The emulsion components may have influenced the palatability of the drinking water, or the herbal contents may have influenced the chickens’ thirst mechanisms and overall health. It was acknowledged that herbal extracts prepared in the form of emulsions with a 5% brown sugar continuous phase provided some advantages, such as the ability to evenly disperse water-insoluble extracts by emulsification process, masking the unpleasant taste of the extracts, easy adjustment of the dosage of the emulsion extracts, and a higher preference to drink the emulsion herbal extracts compared to ordinary water (
Fig. 3). Consequently, the total consumption of drinking water has increased. According to Crowley (2012), emulsions have a number of significant advantages over other liquid forms, including the ability to easily mix compounds that are poorly soluble in water and the ability to partially or completely mask unpleasant tastes or odors (
14). Similarly, other researchers had also reported on the use of herbal extracts as liquid supplements in drinking water for broiler chickens, administered alone or in combination. For instance, there is a report on the effect of turmeric-supplemented water on biochemical and histological parameters (
15), and another on the effect of
Moringa oleifera supplemented water on broiler growth and health (
16).
The current study demonstrated that HEEtreatment groups had significant differences in organ weights and the overall weight of internal organs (p<0.05). The observed variations in liver and heart weights among different treatments indicate that dietary supplements such as honey, turmeric extract, and moringa leaf extract have unique impacts on organ growth. The observed elevation in liver weight among the groups administered with turmeric extract suggests that it has a beneficial effect on liver size and function, possibly attributable to its established antioxidant and anti-inflammatory characteristics. These features are known to promote liver health and metabolic function.
In contrast, the impact of HEE treatments on heart development indicates that these emulsions might improve cardiovascular function, possibly due to the synergistic effects of their bioactive constituents. The inclusion of honey, renowned for its cardiovascular advantages, combined with moringa and turmeric extracts, which possess anti-inflammatory and metabolic-regulating capabilities, may collaboratively enhance the broilers’ heart development and overall health. These findings highlight the possibility of utilizing dietary supplements to regulate the growth and organ function in chickens. The differences in organ weights suggest that each component of the supplement may be able to affect certain physiological pathways, resulting in varying outcomes in organ development.
This finding was consistent with the report of El-Sa’adawy et al., 2023 (
17) where turmeric rhizome powder demonstrated enhanced liver and heart physiological indices, particularly under heat stress. This was also consistent with the studies of Emadi and Kermanshahi, 2006 (
18) and Sudatri et al., 2022 (
15). In contrast, Antyev et al., (2020) demonstrated that 0.1%
Moringa oleifera leaf supplementation in the meal had no significant effect in the relative weights of the kidney, lungs, liver, gizzard, and length of the intestine (
19). Additional research is necessary to clarify the exact processes to examine the ways how these supplements influence organ size and function. Furthermore, it is important to determine the most effective combination and dosages that maximize health advantages while minimizing any potential adverse effects.
The current study demonstrated a significant effect on the reduction of abdominal fat percentage relative to live weight among the treatment groups, as illustrated in
Table 5 and
Fig. 4. The data revealed a consistent trend of decreasing abdominal fat from T3 up to T5 group. There was a non-significant increase in fat content in T2 (moringa leaf extract treatment) compared to the control (drinkable water). These findings suggest that moringa leaf extract alone does not have a notable impact on reducing fat content. However, more complex treatments that included additional components, showed a marked reduction in abdominal fat percentage, indicating that the combination of supplements might have a more pronounced effect on fat metabolism. This highlights the potential for synergistic interactions between different bioactive compounds when used together, which may enhance their overall efficacy in reducing fat deposition in broilers. Nevertheless, contrasting findings have been presented by other researchers, such as Cui et al. in 2018 (
20), who observed a linear and quadratic decrease in abdominal fat (p<0.001) with the addition of
Moringa oleifera leaves to the diet. This was consistent with the findings of Sangkitikomol et al. in 2014 where it was hypothesized that
Moringa oleifera reduces the abdominal fat when a specific dosage is added as a supplement to broiler feed (
21).
On the other hand, the introduction of turmeric extract (T3) and the combination of moringa and turmeric extracts (T4) exhibited more significant effects in decreasing abdominal fat. This indicates that turmeric extract plays a vital role in lowering fat. The most significant reduction in abdominal fat was reported in T5, where a combination of moringa leaf extract, turmeric extract, and honey was used. This implies on the possibility of a synergistic effect, rather than individual effect of each component. Turmeric rhizomes contain curcumin which has antioxidant characteristics and can enhance digestion, lipid, carbohydrate, and protein metabolism through the stimulation of bile and pancreatic fluid flow which contain high levels of digestive enzymes (amylase, lipase, and protease) (
1, 8, 17, 22). Similar findings on the effect of curcumin have been reported in several other studies (
2, 18, 23).
The moringa leaf is a rich source of carotenoids, vitamins, minerals, amino acids, alkaloids, and flavonoids, as well as a rare combination of phenolic compounds (zeatin, quercetin, kaempferol, apigenin) and many other phytoconstituents (
9, 10). They are a natural substitute for antibiotics, enhancing immune responses, influencing the structure of cecal microbes, acting as growth promoters, and improving the performance of broiler chickens (
11, 12, 24).
The significant reduction in abdominal fat, especially in treatments T4 and T5, has important implications for the poultry industry, particularly for the development of diet formulations that aim to reduce abdominal fat in broilers, which is a significant quality and health parameter. However, further research should focus on understanding the mechanisms by which these extracts and honey contribute to fat reduction, their long-term effects, optimal dosages, and potential impacts on other health parameters of broilers.
In contrast, the mean carcass weights (g/bird) in the treatment groups were similar, with no significant differences. This implied that the various emulsion treatments had no significant effect on the broiler’s average carcass weight. The highest carcass percentage was observed in T5, likely due to Moringa leaf extract, turmeric extract, and 10% honey in the HEE. Several factors are to consider in this result, including the dosage of the administered extract, which may be inadequate or need to be increased; the stress levels experienced by the broilers during their growth period; genetic factors and the specific species of the broilers; and the optimal combination of extracts. Additional research is required to examine and enhance the utilization of extracts at different dosages that impact the carcass.
Several other studies that have examined the impact of turmeric extract on broiler carcasses and have reported similar results. For instance, Paredes in 2022 and 2023 (
25, 26) reported that adding turmeric rhizome and marigold to the diet did not significantly alter the weight of broiler carcasses, similarly as Kennedy et al. in 2020 (
27). However, this contradicted the results of Emadi in 2006 (
18), who demonstrated that turmeric extract significantly increased the carcass weight of broilers, which was consistent with the findings of Utami et al. in 2020 (
28).
Additionally, the impact of moringa leaf extract was examined by Nduku et al. in 2020 (
29), who observed no statistically significant variation (p>0.05) in carcass weight; this finding was similar to that of Ologhobo in 2014 (
30). However, several other studies, including those conducted by David et al. in 2012 (
31), presented divergent findings. Their research indicated that supplementation with moringa leaf meal had a significant impact (p<0.05) on carcass weight. Similar findings were reported by Zhang et al. in 2023 (
32) and by Alshukri et al. in 2018 (
33).
Despite the non-significant differences of the treatments on the carcass, the extracts did not have adverse effect on growth performance in broilers. Higher concentrations of HEE in chicken feed may be tested in future trials. Furthermore, the current study demonstrated that HEE in different concentrations had significant effect on lower belly fat and internal organs weight rather than on the whole carcass weight. Age, sex, slaughter weight, body size and conformation, fat, quality and quantity of feed, and breed, have been reported as factors that may impact carcass weight (
34).
HEE had no significant effect on live weight, feed intake, or FCR (
Table 6) which is compliant with findings of other studies. Supplementation of garlic powder and turmeric powder did not significantly affect feed intake, live weight, or FCR (
35). Similar findings have been reported with turmeric rhizome powder (
26, 36). No significant effect has been observed with supplementation of
Moringa oleifera on FCR, feed intake, or live weight (
37, 38).
The T2 treatment exhibited significantly higher growth and FCR at the fourth week. However, no significant differences were observed between the treatment groups at the fifth week. It is crucial to acknowledge that the efficacy of treatments might fluctuate over time and be influenced by multiple factors. Additionally, higher feed consumption is not always correlated with a proportional increase in the live weight.
Several limitations in this study must be acknowledged. Future research would benefit from a wider sample size and a more significant scale to obtain more optimal and accurate results. It should be noted that the sample size used in this study may be too limited to generalize our findings if applied to a larger sample size. However, we can confirm that the statistical analysis has been performed with appropriate consideration of the available data to obtain valid and accurate results. These findings have important implications for chicken farming, notably for improving feed formulas and treatment procedures to improve growth efficiency. This study offers a framework for future research to optimize poultry feed and treatment procedures on a larger scale, aiming to enhance broiler production efficiency.
CONCLUSIONThe present study demonstrated that the HEE formulations were the most preferred by broiler chickens and significantly influenced the weight of the internal organs and the percentage of abdominal fat. However, the percentages of carcasses and the FCR remained unaffected. Consequently, further research is advised to optimize the formulation and dosage, with the objective of maximizing growth benefits while improving efficiency.
CONFLICT OF INTERESTThe authors declare that they have no known conflict of interest in the conduction of the current study.
ACKNOWLEDGMENTSThis research was funded by Universitas Sumatera Utara in accordance with the contract of TALENTA Research Implementation of Universitas Sumatera Utara for 2019 No: 4167/UN5.1. R/PPM/2019 dated April 1st, 2019.
AUTHORS’ CONTRIBUTIONMM conceptualized and designed the study. The acquisition of data was BEP performed the data analysis and SEN interpreted the data.

 10.2478/macvetrev-2025-0016
10.2478/macvetrev-2025-0016









