The composition of animal feed can promote microorganism development. Microorganisms in feedstuffs can be saprophytic, pathogenic, conditionally pathogenic, or toxic (
3). Although most bacteria in feed are unlikely to be hazardous, pathogens may still be present. Various bacterial pathogens, such as
Listeria, Clostridia, pathogenic
E. coli, and
Salmonella, have been identified in animal feed (
4). Microbial contamination of feed can occur from various sources (
5) such as airborne transmission, water sources, and feed exposure during harvesting, processing, and storage (
6).
The diversity of environmental sources contributes to a variety of bacteria that contaminate animal feeds. Factors, such as moisture, temperature, feed type, aerobic, and anaerobic conditions, raw material properties, feed pH, feed supplements, storage conditions, and feed decomposition products, affect bacterial growth and proliferation (
4,
7).
Consequently, animal feed may pose an epidemiological risk to animals, economic losses to feed manufacturers and farmers, and, owing to its zoonotic nature, a possible risk to consumers.
Chemical hazards in feed
Heavy metals
According to the World Health Organization (WHO), arsenic (As), lead (Pb), mercury (Hg), and cadmium (Cd) are four of the ten chemical food-related hazards that pose the greatest threat to public health (
8). Lead, Cd, and As are environmental contaminants that pose serious risks to both humans and animals (
9,
10). Furthermore, they have extensive applications in industrial goods such as pesticides, pigments, crystal glass manufacturing, and plumbing (
9,
10). Typically, Pb accumulates in the kidneys, bones, and liver because of its slower excretion from organisms than other heavy metals (
9). Acute or long-term exposure to Cd can negatively impact animal performance due to histopathological changes and damaging effects on the liver and kidneys, among other effects (
10). The tissues of liver, kidneys, lungs, and gastrointestinal system, rapidly accumulate As (
9). Various industrial applications frequently utilize highly hazardous Hg, which contaminates the environment (
10). Overconsumption of Hg may lead to abnormalities in the neurological, hepatic, and renal systems, thus causing adverse effects on animal’s health (
11). Exposure to heavy metals is inevitable in both animals and humans. Control of animal feeds is crucial to ensure that they are exposed to safe levels of As, Pb, Hg, and Cd, thereby minimizing the negative effects of acute or chronic exposure.
Mycotoxins
Mycotoxins are a group of secondary metabolites produced by fungi, mostly from the genera
Aspergillus,
Penicillium,
Fusarium,
Claviceps, and
Alternaria. They are a major health hazard to both people and food-producing animals, and are believed to cause significant financial losses in feed and food production (
12). Ochratoxin A (OTA), zearalenone (ZEN), and aflatoxins (AFs) are among the most significant toxins due to their occurrence and detrimental effects on human and animal health (
13). Cereals are the primary components of the compound feed products. They are estimated to have a prevalence of 60–80% mycotoxin contamination, which is further transferred into the feeds (
13).
In addition to pH level, water activity (aw), and ambient temperature, crucial factors influencing mold growth and mycotoxin production (
12), the climate change has been identified as a major risk factor contributing to mycotoxin contamination in crops and associated feed products. Beyond increased mycotoxin contamination, climate change has led to a worldwide geographical redistribution of mycotoxins in crops, thereby raising concerns about mycotoxin contamination in various foods and feeds (
12,
14).
Chronic exposure to mycotoxins may have a variety of harmful effects, including altered DNA and RNA synthesis, growth suppression, pulmonary oedema, tissue damage, hormonal imbalances, and carcinogenic effects (
13). The observed effects can differ between species depending on their weight and size; hence, ruminants are less sensitive than pigs and poultry (
13). Due to these factors, mycotoxins are seen as a far more serious threat to farm animals’ health than feed additives or pesticide residues. After ingestion of contaminated feed by food-producing animals, animal-derived foods, such as milk, milk products, meat, meat products, and offal, may contain a notable presence of mycotoxins (
15,
16,
17,
18).
Coccidiostats
Modern intensive husbandry practices lead to significant financial losses due to the high incidence of coccidiosis, particularly in poultry production. Currently, anticoccidial feed additives are the most effective method for controlling coccidiosis. The European Union has approved 11 coccidiostat substances as feed additives, primarily used in chickens, turkeys, and rabbits to prevent coccidiosis in one or more animal species (
19). Based on their main biological activity and chemical structure, these compounds can be classified into two groups: non-ionophoric compounds have a wide range of structures, including robenidine, decoquinate, nicarbazin, diclazuril, and halofuginone, while ionophoric carbocyclic polyethers include monensin, lasalocid, salinomycin, narasin, maduramicin, and semduramicin (
20).
During the production of feed medicated with coccidiostats, accidental transfer of these substances from targeted to non-targeted feed may occur (
19), posing hazardous effects on non-targeted livestock resulting in undesired residual levels in the food (
21). Moreover, the presence of coccidiostats in feed, combined with other feed additives, may result in pharmacological interactions that harm animals (
21). Therefore, the national legislation in North Macedonia sets the maximum permitted carry-over levels to manage unavoidable crosscontamination (
22).
The primary aim of this study was to evaluate the animal feed safety in North Macedonia in the period between 2018 and 2022, based on microbiological and specific chemical hazards assessment such as lead, cadmium, arsenic, mercury, aflatoxin B
1, ochratoxin A, zearalenone, and 11 regulated coccidiostats. Additionally, the study aimed to evaluate the feed compliance with national feed safety standards. These findings are expected to provide critical insights for improving feed monitoring systems, strengthening regulatory enforcement, and safeguarding both animal and human health across the feed-to-food chain. Furthermore, this information can contribute in raising awareness among non-professionals, healthcare organizations, and agricultural stakeholders.
MATERIAL AND METHODS
Microbiological analysis material and methods The National Rulebook for general and specific demands on animal feed safety (
23) sets the criteria for the evaluation of feed safety in North Macedonia. The samples were collected as part of the official feed monitoring program.
To assess the microbiological quality and safety of the feedstuffs, 598 feed samples were analysed from 2018 to 2022. As mandated by the Rulebook, the samples were divided into four categories (raw feed materials of plant origin, poultry feed, swine feed, and cattle feed) and the analyses determined the total number of mesophilic microorganisms, yeasts, molds, and the presence of
Salmonella and sulphite-reducing clostridia (
23).
The total bacterial count was evaluated in the animal feed samples using the reference method (ISO 4833-1:2013) (
24). Total yeasts and molds were evaluated according to ISO 21527-2:2008 (
25), sulphite-reducing clostridia according to ISO 15213:2003 (
26), and
Salmonella spp. according to ISO 6579-1:2017 (
27).
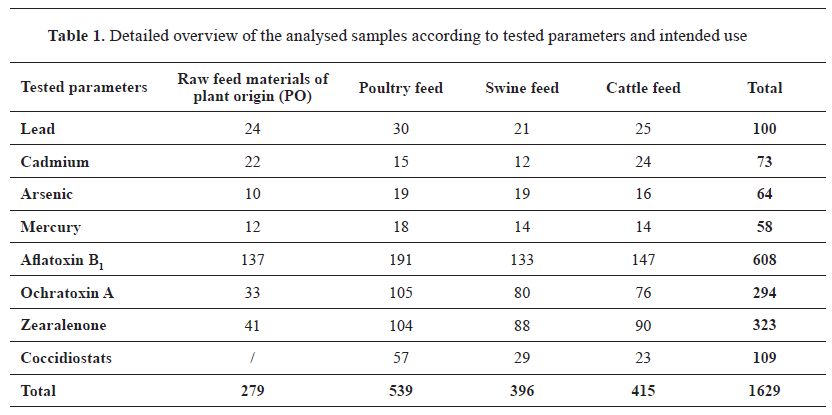
Samples for chemical hazards analysisA total of 1,629 feed samples were collected for chemical hazard analysis from farms, feed mills, and feed business operators between the period 2018-2022. Each sample was obtained from a separate batch with an approximate weight of 500 g. All samples were stored at -20 °C until they were processed and examined. Raw cereal materials or cereal-based complete feeds were analyzed. All samples were tested for moisture content using the standard feed methodology (
28). A detailed breakdown of the tested samples for chemical hazards according to the feeding purpose is presented in
Table 1.
 Analysis of heavy metals
Analysis of heavy metals
The atomic absorption technique was utilized to analyze heavy metals in feeds with electrothermal atomic absorption spectrometer (ETAAS) model AAnalyst 600 (Perkin Elmer, Waltham, Massachusetts) for Pb, Cd, and As, and the cold vapor atomic absorption spectrometer (CVAAS) model FIMS 100 (Perkin Elmer, Waltham, Massachusetts) for Hg. The sample was mineralized by pressure digestion following EN 14084:2003 (29) using a high-performance microwave digestion system (Ethos Up, Milestone Srl, Sorisole, Italy). The analysis of Pb, Cd, and As was performed according to EN 14084:2003 (
29), following the prescribed temperature and time programs for the graphite furnace at selected wavelengths for each element. The Hg analysis was performed according to EN 13806:2002 (
30). Certified reference materials (CRM) solutions of Pb, Cd, As, and Hg (all purchased from Carl Roth GmbH, Karlsruhe, Germany) were used for quantification.
The methods were validated, and the determined limits of quantification (LOQ) for the feed samples were 0.010, 0.004, 0.003, and 0.001 mg/kg for Pb, Cd, As, and Hg, respectively. The linearity of the method was higher than 0.999, precision was <15%, (expressed as the relative standard deviation, RSD), and accuracy was in the range of 90%–110%.
Analysis of mycotoxins
We conducted HPLC-FLD analysis for AFB
1, OTA, and ZEA using a Perkin Elmer (PE) chromatographic system that included a binary pump (PE LC-250), manual injector (PE Rheodyne 7125), and fluorescence detector (PE LC-240). A Kobra® cell (R-Biopharm Rhône) was used for electrochemical derivatisation to enhance the AFB
1 signal. Purification of the sample extracts employs specific immunoaffinity columns: Aflaprep®, Ochraprep®, and Easi-Extract® Zeralenone, purchased from R-Biopharm Rhône, Glasgow, Scotland. A nitrogen evaporator (OA-Heat, N-Vap 116, Organomation, USA) was used to concentrate the OTA and ZEA extracts.
HPLC-grade reagents (water, methanol, and acetonitrile) were purchased from Carlo Erba Reagents (France); benzene, KBr, and NaCl were purchased from Sigma-Aldrich (USA); and 65% HNO
3 , glacial acetic acid, and phosphate buffer solution (PBS) were purchased from Merck (Darmstadt, Germany). The calibration standards AFB
1 (1068 ng/mL), OTA (50 μg/mL), and ZON (50 μg/mL) were purchased from Supelco (Sigma-Aldrich, USA). We performed chromatographic separation isocratically at ambient temperature on RP C18 columns with the following characteristics: 250 mm x 4,6 mm, 5 μm for AFB
1, and 150 mm x 4.6 mm, 5 μm for OTA and ZEA (products of Agilent, Santa Clara, CA, USA). The analytical methods were validated and the LOQs were 0.015, 0.12, and 9.5 μg/kg for AFB
1, OTA, and ZEA, respectively.
The sample preparation and performance characteristics of the applied methods have been previously published elsewhere (
31).
Analysis of coccidiostats
The coccidiostats were analysed by ultrahigh- performance liquid chromatography-tandem quadrupole mass spectrometry (UHPLC-MS/MS) on an ACQUITY I-class UHPLC and Xevo TQ-S system (Waters, Milford, MA, USA) in multiple reaction monitoring (MRM) mode. The mass spectrometer conditions were optimized for 11 analytes using standard solutions prepared from neat substances: decoquinate, monensin, nicarbazin, robenidine (CPA Chem, Bulgaria), diclazuril, lasalocid, maduramicin, salinomycin (Sigma Aldrich, St. Louis Missouri, USA), halofuginone, semduramicin (HPC standards, Borsdorf, Germany), narasin (USP reference standard, Merck Darmstadt). The optimal conditions for the detection of all analytes were: capillary 1.0 kV, cone 25 V, source temperature 150 °C, desolvation temperature 500 °C, desolvation gas nitrogen 800 L/h, and cone gas 50 L/h.
Sample preparation was performed according to the manufacturer’s published method with some modifications (
32). The samples were purified using OASIS HLB 200 mg solid-phase extraction columns (Waters, Milford, MA, USA). Before injection into the UHPLC-MS/MS system, the extracts were diluted and filtered through a RC syringe filter (Sartorius, Goettingen, Germany). The mobile phase consisted of LC-MS grade water with 0.1% formic acid (mobile phase A) and acetonitrile-methanol (50:50) with 0.1% formic acid (mobile phase B) (Merck, Darmstadt). Chromatographic separation was performed using an analytical UHPLC column BEH C18, 100 mm x 2.1 mm x 1.7 μm (Waters, Milford, MA, USA).
Matrix-matched calibration standards were used for quantification and the correlation coefficient obtained was >0.95. The determined LOQs were 0.009 mg/kg (halofuginone, maduramicin, diclazuril), 0.09 mg/kg (decoquinate, semduramicin), 0.1 mg/kg (robenidine, salinomycin, narasin), 0.3 mg/kg (monensin, lasalocid, nicarbazine). The precision was below 11%, expressed as RSD, and the accuracy was in the range 70-120%.
Ethical statement
The results included in this research are published according to the approval issued by the Food and Veterinary Agency of the Republic of North Macedonia (No. 02-3564/2 from 17th January 2025).
Data calculation and statistical analysis
The measured values from the heavy metals, mycotoxin’s and coccidiostat’s analysis were normalised to a moisture content of 12%. Descriptive statistics were performed for the heavy metal and mycotoxin data, calculating the mean values from the positive samples (over LOQ), mean values from the total samples, and median and maximum values for the data sets. To summarize the central tendency and variability of the dataset distribution, we calculated the 1
st and 3
rd data quartiles.
The obtained datasets were statistically processed by approximating the analyte values for the determined below the LOQ to zero. To estimate the significance between datasets, statistical analysis of variance (ANOVA) was performed using Statistica software version 14 (StatSoft, STATISTICA Software), applying a significance level of p=0.05. We performed the descriptive statistics using Microsoft Excel 2016 MSO (16.0.4312.1000).
RESULTS
Heavy metals
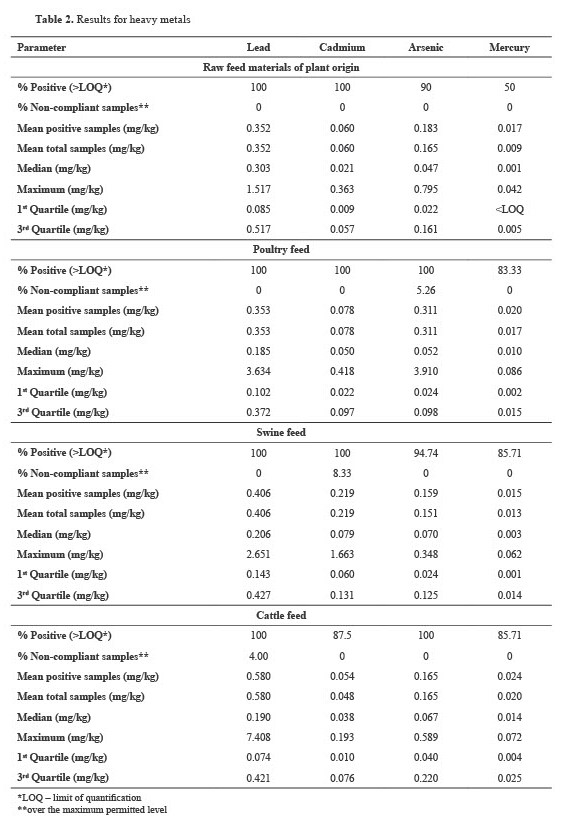
Table 2 displays the results of the heavy metal analysis, representing the positivity and noncompliance rates, mean concentrations of the quantified metal levels, and descriptive statistics.
The descriptive statistics presented in
Table 2 reveal positive median values for all tested parameters in all feed samples, which was expected considering the high positivity rate. The lower value (1
st quartile) for all tested mycotoxins across all feed types, except Hg in raw feed materials of PO, was above the LOQ, meaning that all results between the lowest and middle values were quantifiable. The upper 3
rd quartile, indicating the data distribution between the middle and highest numbers in the dataset, was above the LOQs for all analytes and all feed types. The statistical test for significance (p
>0.05) did not reveal any differences among the various feed types for any of the tested parameters.
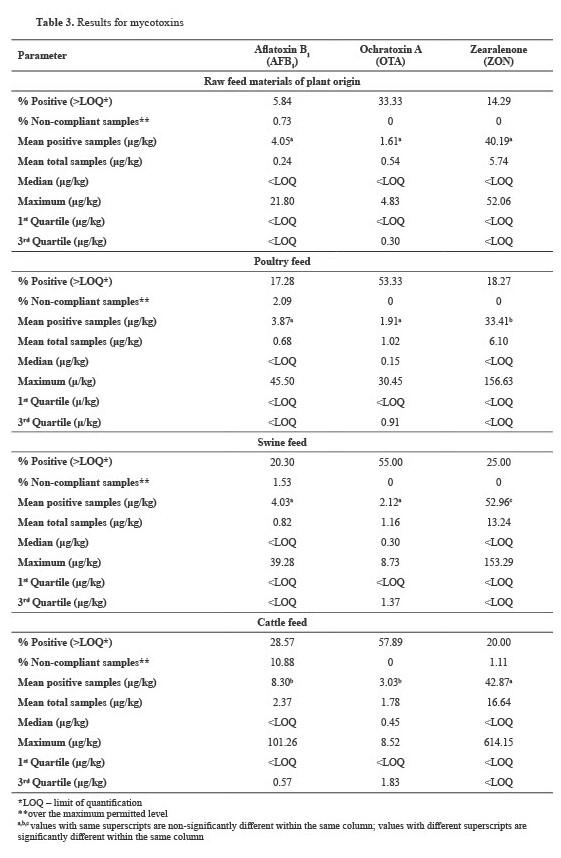
Mycotoxins
Table 3 summarizes the results obtained from mycotoxin testing of the samples presented in
Table 1. The most prevalent finding across all feed types was OTA; the contamination rate for this mycotoxin ranged from 33.33% to 57.89%. Statistical differences in OTA results were ascertained between raw feed materials of PO and swine and cattle feed. The statistical test for significance for AFB
1 encountered differences between the datasets for PO feeds and cattle feeds, poultry and cattle feed, and swine and cattle feed. The ZEA-tested samples had contamination rates ranging from 14.29% for PO feed to 25.0% for swine feed. We observed statistical differences between swine feed and raw feed materials of PO as well as between poultry and swine feed. The statistical differences between the mean positive values are adequately marked in
Table 3.
The mean mycotoxin levels in all feed types were higher than the LOQ of the applied analytical methods (
Table 3). On the other hand, the median values for AFB
1 and ZON across all feed types were below the LOQ of the corresponding methods, whereas we observed positive medians for OTA in poultry, swine, and cattle feed. The values of the lower quartile (1
st quartile) for all tested mycotoxins and feed types were below the LOQ. Conversely, for AFB
1 in the raw feed materials of PO, poultry, and swine feed and ZON in all feed types, the upper 3
rd quartile was less than the LOQ. For OTA, the 3
rd quartile values for all feed types were above the LOQ of the method. The 3
rd quartile values below the LOQ indicated that the datasets were highly left-censored.
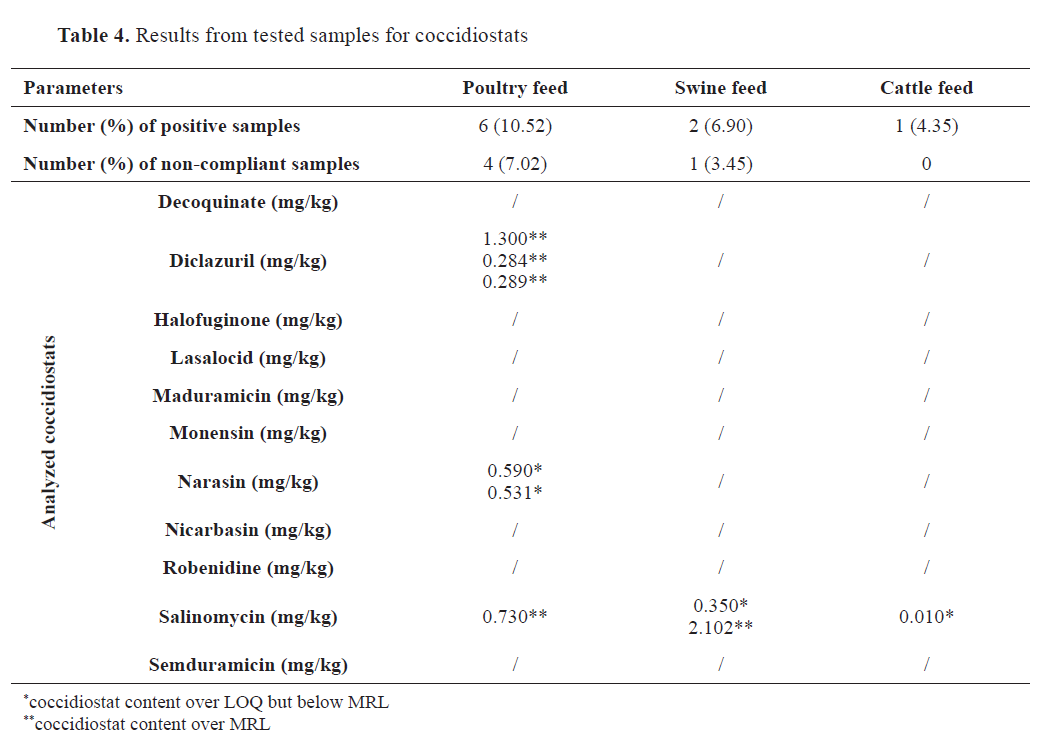
Coccidiostats
A total of 119 feed samples were tested for 11 coccidiostat substances at the carry-over level: decoquinate, monensin, nicarbazin, robenidine, diclazuril, lasalocid, maduramicin, salinomycin, halofuginone, semduramicin, and narasin. From the tested coccidiostats, positive findings over the method LOQ were obtained only for diclazuril, narasin, and salinomycin, mostly in poultry feed (
Table 4). We did not perform statistical analysis because of the low positivity rate.
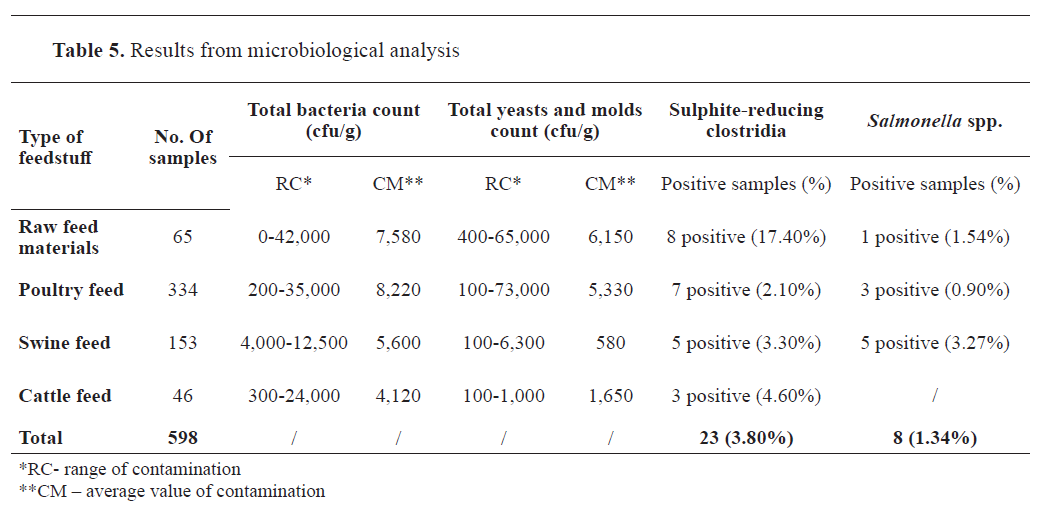 Microbiological results
Microbiological resultsThe results are expressed according to the guidelines set up in the National Rulebook and associated ISO standards (total bacterial count, the total number of yeasts and molds, and the presence of sulphite-reducing clostridia per gram, and the presence of
Salmonella per 50 g).
Table 5 provides the microbiological characteristics of the different feedstuffs evaluated. Of the 598 feed samples, 23 (3.8%) were positive for sulphite-reducing clostridia, and eight (1.34%) tested positive for
Salmonella spp.
The average prevalence of
Salmonella spp. in the feed materials analysed between 2018 and 2022 was 1.54% in raw feed materials of plant origin, 0.90% in poultry feed, and 3.27% in pig feed. None of the 46 cattle feed samples tested positive for
Salmonella spp.
DISCUSSION
Heavy metals
Heavy metals are toxic substances that are not degradable or metabolized by animals after ingestion. Lead in bones and Cd in animal kidneys are examples of how some of them deposit and irreversibly bind to body tissues (
33).
The results from this study (
Table 2) suggest that the heavy metal levels in the tested feed are generally below the ML according to the legislation (
22). Among the tested samples, we found one Pb result for cattle feed (7.408 mg/kg), one As result for poultry feed (3.910 mg/kg), and one Cd result for swine feed (2 mg/kg) that exceeded the maximum levels (ML) (
22). However, the prevalence was very high (50%–100%) for all elements in all feed types.
Lead was the most abundant chemical element, with a positivity rate of 100% across all feed types, indicating that all findings exceeded the LOQ of the applied methodology. Accounting for the overall Pb results for all feed types, our findings are consistent with those of Adamse et al. (2017), ranging from LOQ up to 3.5 mg/kg. Our Pb results differ significantly from those published by Iqbal et al. (
34), with levels from 0.93 to 8.87 mg/kg. Korish and Attia (
35), on the other hand, disclosed Pb levels in poultry feed up to 4.14 mg/kg, slightly higher than our maximum concentration (3.634 mg/kg). Data published by Hejna et al. (
36), Wang et al. (
37), and Adamse et al. (
38), as well as our findings, unveiled Pb content bellow ML, suggesting that swine and cattle feed did not pose any apparent risk for intensive production systems. Surprisingly, the results published by Zhang et al. (
39) did not detect Pb in any of the tested samples. They were unexpectedly below the LOD considering the abundance of Pb in the environment and its extensive industrial use (
9).
With exception of one swine feed sample with a Cd level of 1.663 mg/kg, its concentrations in the current study were well below the legal limit of 1 mg/kg (
22). The Cd results (
Table 2) are consistent with those published by Adamse et al. (
38), who reported levels from LOQ to approximately 1.8 mg/kg. Furthermore, Korish and Attia (
35) revealed a maximum Cd level of 0.111 mg/kg in poultry feed, which was lower than the results of this study (
Table 2). Cadmium levels in cattle, poultry, and swine feed were significantly higher in published results for Northeast China (
39). Iqbal et al. (
34) did not report any positive findings for Cd in the tested feeds, which is contrary to the findings of our study and the previously mentioned studies.
The As findings from our study are in line with the reported values by Hejna et al. (
36), with a mean value of 0.38 mg/kg for swine and 0.09 mg/kg for cattle feedings. Zhang and co-workers (
39) have reported significantly higher amounts for cattle, poultry, and swine feeds (up to 6.12, 6.42, and 10.95 mg/kg, respectively). Eliot et al. also reported high levels of As that exceeded ML, with contamination ranging from 14 to 27%, depending on the type of feed (
33). Adamse et al. reported low findings in complete feed, with an average of 0.1 mg/kg (
38). Increased As contamination in feed may arise from its high presence in soils and rocks as well as its extensive use in various plant protection products (
10).
Approximately 85% of the tested poultry, swine, and cattle feeds contained Hg; however, the maximum determined amounts were lower than the ML value of 0.1 mg/kg (
22). The levels reported in a study by Iqbal et al. were all below the method’s LOD (
34). In contrast, a study by Adamse et al. (
38) revealed that 2% of non-compliant samples exceeded the level of 0.1 mg/kg. Generally, Hg data are scarce in feed types; fish meal, seaweed, and other marine algae are the main subjects of published results (
38).
Mycotoxins
The continental European region shows the most pronounced evidence of the impact of climate change on mycotoxin occurrence in cereals and feeds due to increased average precipitation and notable growth in rainfall frequency, whereas the Mediterranean region experiences more frequent and long-lasting droughts (
12,
14). Therefore, our study focused on recently published mycotoxin findings from European regions. The results from our study (
Table 3), as well as recent studies published by other authors (
13,
40) or presented in systematic review articles (
41), disclosed notable amounts of mycotoxins in the tested feeds.
Aflatoxin B1
Based on the data presented in
Table 3, we can conclude that the non-compliance rate for AFB
1 was significantly higher across all feed types: 0.73% for raw feed materials of PO, 1.53% for swine feed, 2.09% for poultry feed, and 10.88% for cattle feed. Moreover, of the 16 non-compliant cattle feed samples, 14 (87.50%) were intended for dairy cow feed, with values ranging from 5.60 to 36.41 μg/kg.
Due to metabolic transformation to aflatoxin M1 (AFM1), AFB
1 ML for cattle feed is very strict, and for lactating cows, it was set at 5 μg/kg (
22). Two samples of feed intended for mature cattle exceeded the ML value of 20 μg/kg. The AFB
1 positivity rate for cattle feed (
Table 3) was similar to those reported in studies from Spain (33%) and Turkey (26%) (
41). Other studies from Spain (
13) and Italy (
40) revealed lower positivity rates of 12% and 14%, respectively, which is more similar to the reviewed data for Italy (5.8%) and Poland (12%) (
41). The maximal AFB
1 levels in cattle feed in our study (
Table 3) are comparable to the findings of Sdogati et al. (
40), estimated at 104.5 μg/kg; whereas another study from Italy showed a higher value of 232 μg/kg (
41). Studies conducted in Spain (
13,
14) reported significantly lower AFB
1 maximum of 6.5 μg/kg and <2 μg/kg similar to studies from Turkey (6.89 μg/kg) and Poland (1.31 μg/kg) (
41). Regarding swine feed samples, AFB
1 levels over 2 μg/kg were not detected in Spain and Poland (
41). Muñoz-Solano et al. have reported a 7% positive rate with the highest detected amount of 6.2 μg/kg (
13). The AFB
1 incidence in poultry feed was low (
Table 3), similar to two independently published results for Spain (
13,
41) and one from Italy (40), estimated at 13%, 8%, and 11%, respectively. The maximum determined AFB
1 value in our study is 45.5 μg/kg, which is significantly higher than levels of 6.9 μg/kg (
13), 29.1 μg/kg (
40), and <2 μg/kg (
41). Unlike our findings (
Table 3), the aforementioned studies did not reveal any samples exceeding ML. The raw feed materials of PO are less susceptible to AFB
1 contamination (
40), which could explain the low incidence observed in this study (
Table 3). A study from Spain (
41) reported no positive data for raw feed materials of PO; however, a study by Sdogati et al. (
40) reported a 0.2% incidence, with a maximum amount of 15.6 μg/kg.
The discrepancies between some of the aforementioned AFB
1 findings and our study (
Table 3) are probably a result of the variability of the implemented management practices in crop and animal production throughout the European regions towards climate change. The positivity rate was also dependent on the LOD and LOQ values, which for our methodology (
31) appeared to be lower than those reported by other authors (
13,
40,
41).
Ochratoxin A
The results from the tested feed samples for OTA did not reveal an exceedance of the ML values according to the regulative (
22); the highest determined level was 30.45 μg/kg for mature poultry. Considering the ZON results, only one finding for cattle feed (614.15 μg/kg) exceeded the ML value (
22).
The unveiled OTA incidence for cattle feed (
Table 3) was closest to that reported for Spain (33%) and Turkey (26%), but significantly higher than other findings for Spain, estimated at 6% (
41), and Italy being less than 1% (
40). However, studies from Spain and Turkey reviewed by Santos Pereira et al. (
41) reported significantly higher incidences of cattle feed samples (80% and 95.45%, respectively). Concerning poultry feed, two independent studies from Spain (
13,
41) reported positivity rates of 5% and 11%, respectively. Studies from Poland, Spain, and Norway have reported positivity rates ranging from 40 to 80% (
41). The maximum detected levels in our study are in line with research from Spain (<25 μg/kg) (
41) and close to the 42 μg/kg reported in Italy (
40). The range of the quantified OTA levels for Poland, Spain, and Norway varied from 1.44 μg/kg up to 88 μg/kg (
41). Reports on swine samples revealed OTA contamination rates of 7% and 33% in Spain (
13,
41) and 35% in Italy (
40), respectively, with no exceeding the ruled-out MLs. The positivity rate in our study was 55% (
Table 3), probably due to the lower LOQ (
31). The OTA incidence in tested feed materials of PO from Italy was below 1%, which could be attributed to several factors, such as low contamination of cereals with OTA-producing molds and higher method LOQ resulting in left-censored data (
40).
The higher OTA positivity rate and higher maximum levels found in North and North-East European regions imply that increased average temperatures probably have become favorable for the development of OTA-producing
Aspergillus and
Penicillium species (
12).
Zearalenone
A study reporting ZON results for Spanish cattle feeds showed an incidence of 49%, with a maximal value of 413 μg/kg; 50% incidence and a maximal value of 816 μg/kg for swine feeds; and 66% incidence with a maximum of 489 μg/kg for poultry feed (
13). Another study from the same country reported an incidence rate of 11%, and maximum value of 88.2 μg/kg for cattle feed, 11% for incidence and maximum values were lower than 50 μg/kg for poultry feed, 6% incidence, and a maximum of 14.8 μg/kg for swine feed (
41). Sdogati et al. (
40) reported positivity rates similar to those in our study for cattle, poultry, and swine (20%, 8%, and 25%, respectively). However, the maximum concentrations were in the range 330–1,698 μg/kg. The disclosed data in the review paper by Santos-Pereira et al. (
41) showed a significantly higher incidence for swine feed from Norway (97%), complete feeds from Poland (99%), with maximum concentrations of 217.2 μg/kg and 349 μg/kg, respectively. A study from England (
41) also disclosed a higher incidence than that in our study (39%), with a maximum determined level of 1,431 μg/kg. Unlike AFB
1 which is characteristic of regions with high temperatures and long-lasting drought periods, the optimum temperature for ZON synthesis in crops is approximately 20-25 °C with heavy rainfall (
12). Such climatic conditions caused by rising global temperatures have become more common in central and northern European regions (
12). This could be the main reason for the higher incidence of ZON in the feed samples from Norway, Poland, Hungary, and England. Feeds from regions with long-lasting temperatures over 35 °C such as North Macedonia, are less affected by ZON produced by
Fusarium molds.
Coccidiostats
In nine samples across all feed types, for the substances diclazuril, narasin, and salinomycin, determined levels were higher than the LOQs. Poultry feed showed the highest positivity (10.52%), with four of the nine samples (7.02%) containing coccidiostat residues over the ML (
22). Diclazuril, which was present in three samples, was the most abundant substance, all of which exceeded the ML value of 0.01 mg/kg. One poultry feed sample contained salinomycin at amount exceeding the ML value (0.7 mg/kg). Two poultry feed samples contained narasin levels lower than the ML (0.7 mg/kg) specified in the respective legislation (
22). Two swine feed samples were positive for salinomycin, with one sample surpassing the ML. In cattle feed, there was only one quantifiable result for salinomycin, which was lower than the ML value.
The findings for coccidiostats in this study were consistent with those published by other authors (
42,
43), who concluded that salinomycin was the most frequently detected coccidiostat, followed by narasin. The results of our study did not align with those published by Annunziata et al. (
44) and Moretti et al. (
45), with monensin being the most abundant coccidiostat in the tested feed samples.
The positivity rate for poultry feed (
Table 4) was lower than that reported by Annunziata et al. (44) of 18.5% and Roila et al. (
19) of 16.7%. The overall positivity rate from all tested samples was 7.34%, which is close to that reported in studies that detected coccidiostats in 9% (
45) and 15% (
21) of the tested samples. Roila et al. conducted a study that, depending on the feed type, revealed a similar non-compliance rate, ranging from 1.5% to 16.7% (
19). However, our results disagree with those of other studies that reported significantly higher positivity rates of 72.7% (
42) and 32.4% (
44). Regarding non-compliance, our study revealed that 4.6% of the samples exceeded the regulatory limits (
22). Moretti et al. (
45) reported very similar noncompliant results (5%), while Roila and co-workers reported non-compliance ranging from 1.5% to 6.9% for various feed types (
19). Annunziata et al. found that 11.3% of tested samples were noncompliant (
44).
The reported results for coccidiostats indicate that feed business operators, animal farmers, and feed distributors should be more aware of this problem. Cross-contamination is frequently unavoidable during feed production because of the electrostatic properties of coccidiostat molecules or other factors such as particle size and adhesive strength (
44). Therefore, appropriate preventive measures should be taken, such as thorough cleaning of all feed mill equipment between batches of medicated and non-medicated feed.
Microbiological parameters
Microbial contamination of the animal feed used in North Macedonia between 2018 and 2022 was assessed. To ensure the quality and safety of feed provided to farm animals, microbiological examination of animal feed is crucial (
46).
Although saprophytic bacteria were commonly identified in the examined samples, they are not usually the source of low microbiological quality. The total number of bacteria varied slightly across different feedstuffs. It ranged from 0 to 42,000 cfu/g for plant-origin feed, which had the highest load, and to 12,500 cfu/g for pig feed, which had the lowest load. The parameters for total bacterial count were below the limits prescribed by the Rulebook (
23). However, their presence should not be disregarded as they lower the nutritional value of the substrate by consuming nutrients to meet their metabolic needs (
47).
Various authors have reported the presence of bacterial agents such as
C. perfringens and
Salmonella in animal diets (
48,
49). The two main issues with these infections are that they can infect animals and contaminate meat products with foodborne pathogens that can affect humans (
50).
During a five-year testing period, the prevalence of
Salmonella spp. in feed samples intended for the most significant farm animal species (poultry, pigs, and cattle) in North Macedonia ranged from 0.90% to 3.27%, which is consistent with other authors’ findings of 0.6%-3.5% (
51). Our findings are closely comparable to those of research conducted in European Union countries, which suggests that pig and poultry feeds are the most contaminated with
Salmonella spp. (
52). A plausible reason for the lack of
Salmonella in cattle feed samples could be the small number of specimens examined.
The final feed products may still contain
Salmonella even after the “kill” stage. Feed high in fat and low in water activity could protect
Salmonella from death, allowing it to grow when warmer and moist conditions occur during storage (
53). Additionally,
Salmonella can form biofilms on equipment surfaces, which can contaminate many feed batches (
54).
Anaerobic bacteria in the genus
Clostridium, such as
C. perfringens, are another class of bacteria that the feed industry is concerned about due to their ability to cause disease in animals. According to Maciorowski et al. (
48), clostridial toxins can cause necrotic enteritis.
The findings of this study confirmed the presence of
Clostridium spp. in all types of animal feed, although at lower levels compared to previous investigations (
46,
49). Among the tested feed types, raw feed materials exhibited the highest contamination rate (17.4%), whereas poultry feed exhibited the lowest prevalence (2.1%). These results highlight the persistent risk of
Clostridium spp. contamination in animal feed and emphasize the need for continuous monitoring and preventive measures to safeguard animal health and food safety.
Mycological contamination of the feed materials revealed that 100% of the feed samples did not exceed 10⁴ cfu/g. The total number of molds in the examined samples ranged from 100 to 65,000 cfu/g, which is below the maximum permissible limit set by the National Rulebook. Plant-origin feedstuffs had the highest levels of mold contamination, ranging from 400 to 65,000 cfu/g, whereas pig feed had the lowest levels, ranging from 100 to 6,300 cfu/g.
CONCLUSION
This study evaluated multiannual feed safety data from the food-producing animal sector in North Macedonia, focusing on microbiological hazards, heavy metals, mycotoxins, and coccidiostats. Microbiological analysis of feed samples detected sulphite-reducing clostridia and
Salmonella spp. Mycological contamination remained within the safe limits. Heavy metal analysis showed that significant number of the samples contained Pb, Cd, As, and Hg, although MLs exceedances were rare. Aflatoxin B
1 was present in multiple samples, with highest non-compliance rates in cattle feed. On the other hand, OTA and ZON posed a lower risk; however, substantial number of samples contained OTA above LOQ. Coccidiostat analysis revealed diclazuril, narasin, and salinomycin, exceeding the ML values in five of the tested samples. Poultry feed contained the highest amount of diclazuril, whereas salinomycin peaked in the swine feed.
These findings underscore the need for an upgraded feed safety legislation amid evolving agricultural practices and climate change. Additionally, addressing gaps in the feed safety chain, including monitoring of feed additives and antimicrobial substances, is crucial for protecting animal and public health.
CONFLICT OF INTEREST
The authors declare that they have no financial or non-financial conflict of interest regarding authorship and publication of this article.
ACKNOWLEDGMENTS
We gratefully acknowledge the support provided by the Faculty of Veterinary Medicine-Skopje, Ss. Cyril and Methodius University in Skopje, under the project FVMS-IPR-7, grant No. 0202-359/14.
AUTHORS' CONTRIBUTION
EDS conceived the design, conceptualization, writing, and data processing. BSD and VE carried out the methodology, sample analysis and data processing. LA was included in conceptualization, data acquisition and writing. ZHM reviewed and edited previous versions of the manuscript. GI was responsible for data acquisition and data analysis. DK and AA participated in the practical performance and laboratory analysis. SM was involved in data acquisition, design, conceptualization, data analysis, writing. All authors have reviewed and approved the final version of this manuscript for publication.

 10.2478/macvetrev-2025-0023
10.2478/macvetrev-2025-0023




