This study was designed with the hypothesis that intestinal mucosal epithelial barrier damage in Giardia intestinalis infected dogs could be determined by I-FABP, IAP, TFF-3 biomarkers. The aim of this study was to assess a non-invasive method for detection of intestinal mucosal epithelial barrier damage in dogs caused by Giardia intestinalis with intestinal damage biomarkers.
The study was conducted in the period between January and July 2023. It included 39 dogs admitted at the Small Animal Hospital, Faculty of Veterinary Medicine, Selcuk University. The study protocol was approved by the Institutional Ethics Committee at the Faculty of Veterinary Medicine, Selcuk University (No. 2023/035). A statement had been obtained from all dog owners confirming their consent for the samples to be used for scientific purposes.
The healthy group was comprised of 13 healthy dogs of the same age range (2 months to 4 years). They were admitted at the faculty clinic for routine vaccination. There were 8 male and 5 female dogs. The distribution among breeds was 3 Kangals, 2 Golden Retrievers, 2 Terriers, 1 German Shepherd, and 5 mixed breeds. The dogs underwent a complete physical examination and fecal analysis was conducted to ensure that they were healthy before inclusion in the study. The fecal samples were collected and analyzed in three consecutive days due to the intermittent spread of giardiasis. Samples with no findings of Giardia cysts on the three consecutive analyses, were considered negative.
Dogs with combined infestation confirmed by coproparasitological analysis, including giardiasis and other parasitic diseases (toxacariasis, isosporiasis, etc.), and dogs that were seropositive for Parvovirus or Coronavirus (Asan Easy Test Corona Parvo®, Asan Pharmaceutical, Korea), were not included in the study. In addition, animals treated with antiparasitic drugs for at least 3 weeks prior the study, were not included.
About 5 g of fecal samples were collected directly from the rectum of the dogs and were placed in two different fecal containers. One of the fecal samples was sent to the laboratory at the Department of Parasitology, Faculty of Veterinary Medicine, Selcuk University for coproparasitological analysis (native examination and flotation). The other part was screened for Parvovirus and Coronavirus with a rapid antigen test kit (Asan Easy Test Corona Parvo ®, Asan Pharmaceutical, Korea).
Collection of blood samples
Blood samples from the healthy and
Giardia intestinalis positive dogs were collected from the cephalic vein at the time of admission into 8 mL vacuum serum tubes. The serum samples were centrifuged at 2,000
g for 5 min after clotting. The serum was removed and stored at -80 °C until analysis.
Biomarker analyses
Serum I-FABP, TFF-3, IAP concentrations (Bioassay Technology Laboratory Co., Zhejiang, China) were measured with commercial canine- specific ELISA test kits according to the manufacturer’s instructions. Canine I-FABP commercial ELISA kit (Bioassay Technology Laboratory Co., Zhejiang Chine Cat. No: E0304Ca), Canine TFF-3 commercial ELISA kit (Bioassay Technology Laboratory Co., Zhejiang Chine Cat. No: E0305Ca), and Canine IAP commercial ELISA kit (Bioassay Technology Laboratory Co., Zhejiang Chine Cat. No: E0438Ca) were used for ELISA analyses of biomarkers. The intra-assay coefficient of variation (CV), inter-assay CV, and minimum detectable concentrations (MDC) for biomarkers were <8%, <12%, 0.12 ng/mL for I-FABP, <8%, <10%, 0.29 ng/mL for TFF-3, <8%, <10%, 0.26 ng/mL for IAP, respectively.
Statistical analysis
SPSS 25 (IBM Corp®, 2017, Armonk, NY, USA) statistical program was used to evaluate the data. One Sample Kolmogorov-Smirnov test was applied to evaluate the prerequisites for normal distribution (parametric or non-parametric) of the data. The study data showed parametric distribution and were evaluated using Student’s t-test, they were presented as mean ± SD. The Cohen’s d was calculated to determine the effect size. The power of each analysis was calculated based on Cohen’s d values with a threshold of 0.95. The Pearson correlation test was used to determine the correlation between variables. Statistical significance was considered as p<0.05.
RESULTS
Clinical findings
Dogs with giardiasis included in the study had watery-mucous diarrhea for several days. They had decreased appetite and mild to moderate dehydration.
Biomarker findings
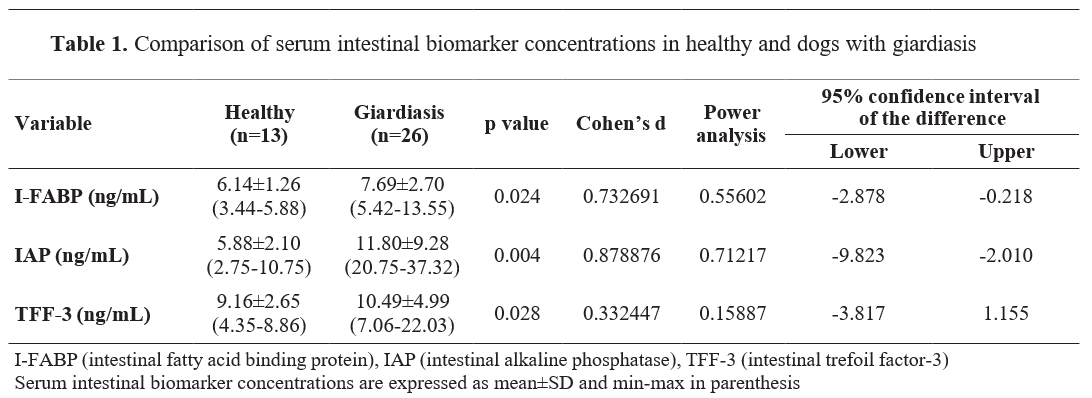
Intestinal biomarker findings of healthy and dogs with giardiasis are presented in
Table 1. Serum I-FABP (Cohen’s d=0.73, 95% Confidence interval (CI): -2.8, -0.2), IAP (d
=0.87, 95% CI:-9.82, -2.01), TFF-3 (d
=0.33, 95% CI: -3.8, 1.15) concentrations were higher in dogs with giardiasis compared to the healthy group (p<0.05). Serum I-FABP concentrations were minimum 3.44 ng/mL and maximum 5.88 ng/mL in the healthy group and minimum 5.42 ng/mL and maximum 13.55 ng/mL in the giardiasis group. Serum IAP concentrations were minimum 2.75 ng/mL and maximum 10.75 ng/mL in the healthy group and minimum 20.75 ng/mL and maximum 37.32 ng/mL in the giardiasis group. Serum TFF-3 concentrations were minimum 4.35 ng/mL and maximum 8.86 ng/mL in the healthy group and minimum 7.06 ng/mL and maximum 22.03 ng/mL in the giardiasis group.
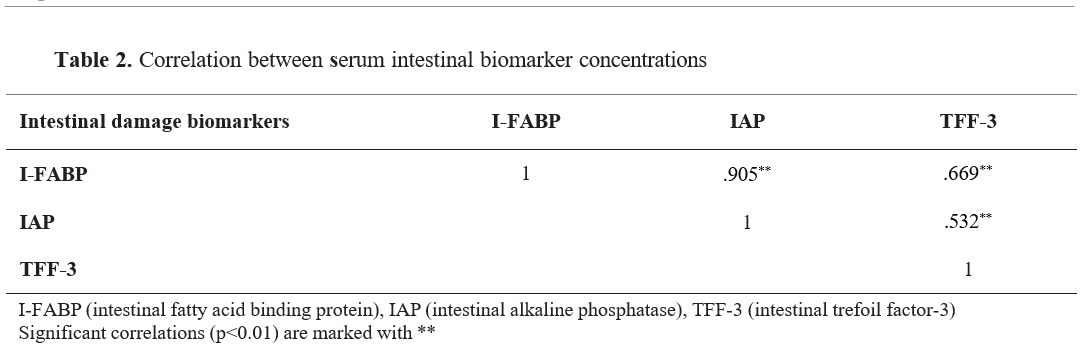
Correlation analysis findings between serum biomarker concentrations are presented in
Table 2. A positive correlation was found between serum I-FABP and IAP (
r=.905), TFF-3 (
r=.669) concentrations (p<0.01).
DISCUSSION
The current study assessed I-FABP, IAP, and TFF-3 as biomarkers of intestinal damage in dogs positive on giardiasis. Our results showed that serum I-FABP, IAP, and TFF-3 levels may be useful diagnostic markers in the evaluation of intestinal mucosal epithelial barrier damage in dogs with giardiasis.
Intestinal fatty acid binding proteins (I-FABP) are cytosolic protein molecules synthesized by enterocytes particularly in the duodenum, jejunum, ileum, and colon (
18). The function of I-FABP in intestinal enterocytes is to support their energetic homeostasis and to transport long-chain fatty acids (
19). In healthy humans and animals, I-FABP is released into the bloodstream at very low or undetectable concentrations, whereas when intestinal mucosal damage develops, it begins to be released into the bloodstream in high concentrations(
20). Increased serum I-FABP concentrations have been reported in humans with inflammatory bowel disease (
21), celiac disease (
22), giardiasis (
23), and acute intestinal necrosis (
24) due to intestinal mucosal damage. Serum I-FABP concentrations in calves have been reported to be higher than healthy calves in cases of atresia coli (
12), neonatal enteritis (
14), and coccidiosis (
15). Additionally, I-FABP is a useful biomarker in determining intestinal damage in dogs with parvoviral enteritis (
13,
25) and isosporiasis (
10). In this study, serum I-FABP concentrations were higher in dogs with giardiasis compared to the healthy group (p<0.05). These results were associated with increased release of I-FABP into the blood due to intestinal mucosal epithelial barrier damage during the life cycle of
Giardia intestinalis (
15,
23).
Intestinal alkaline phosphatase (IAP) is released from the apical microvilli of enterocytes into the intestinal lumen and has been shown to be an important factor in protection from mucosal damage by regulating intestinal surface pH, absorption of lipids, detoxification of free nucleotides and bacterial lipopolysaccharide, and reduction of intestinal inflammation (
26,
27). Intestinal alkaline phosphatase detoxifies bacteria by dephosphorylation and thus plays an anti- inflammatory role during intestinal inflammation (
28). Studies on necrotizing enterocolitis have demonstrated that IAP-enriched nutrition reduces intestinal mucosal epithelial barrier damage and inflammatory cytokine release, confirming the anti-inflammatory and protective effects of IAP in the intestines (
29,
30). Serum IAP concentrations have been reported to be higher in calves with atresia coli (
11), and those with neonatal enteritis (
14) compared to healthy calves. Similarly, serum IAP concentrations in dogs with isosporiasis (10) were higher than those in healthy dogs. Studies in Chron’s disease have reported that low IAP concentrations result in high mucosal damage (
31,
32). In contrast to these studies, serum IAP concentrations have not been shown to be useful in determining intestinal mucosal epithelial barrier damage in calves with coccidiosis (
15). In the present study, serum IAP concentrations were higher in dogs with giardiasis compared to healthy dogs (p<0.05). High serum IAP concentrations in dogs with giardiasis have been associated with intestinal mucosal epithelial barrier damage and increased release to reducing/ healing the damage (
26,
27).
Intestinal trefoil factor is polypeptide secreted by mucus cells in the intestine. These peptides are known to be cytoprotective and promote healing gastrointestinal damage (
33). Elevated concentrations of TFF-3 have been reported in infants with necrotizing enterocolitis (
34), and in humans with ulcerative colitis (
35) in response to mucosal damage. Serum TFF-3 concentration was found to be increased in both dogs with parvoviral enteritis (
13) and calves with neonatal enteritis (14). It was reported that serum TFF-3 concentrations did not differ before treatment in dogs with isosporiasis (
10) and calves with coccidiosis (
15). However, they were increased after the treatment due to increased mucosal damage repair. In the present study, serum TFF-3 concentrations were higher in dogs with giardiasis compared to those in healthy dogs (p<0.05). These results indicate the presence of giardiasis-associated mucosal damage in the intestines. The increased TFF-3 serum concentrations have been additionally associated with its higher release from tissues as a cytoprotective response (
34,
35).
The simultaneous increase in serum concentrations of intestinal damage biomarkers (I-FABP, IAP, and TFF-3) (
Table 1) and their strong in-between correlation (
Table 2, p<0.01) indicate that they are highly associated with the ongoing intestinal damage during giardiasis as a protective response. The present study has some limitations. The high concentration of biomarkers observed in dogs with giardiasis suggests the presence of intestinal epithelial barrier damage and highlights the relationship between diarrhea and intestinal damage. However, for more accurate assessment of the association between biomarker levels, the severity of intestinal damage, and the severity of diarrhea, additional data could be introduced in the overall analysis such as patient follow-up, fecal scoring, and a larger sample size. In addition, the biomarkers of intestinal epithelial barrier damage have been evaluated in numerous studies, but the lack of histopathological confirmation of intestinal damage can be considered as a deficiency. Another limitation is the statistical power of the analyses. Power calculations based on Cohen's d values showed that all comparisons had power values below the recommended threshold of 0.95, suggesting that the sample size may not have been sufficient to detect smaller effect sizes with high confidence. Future studies with larger sample sizes and histopathological validation are recommended to further investigate the relationship between intestinal biomarkers and giardiasis-induced mucosal damage. CONCLUSION In conclusion, I-FABP, IAP, and TFF-3 concentrations were higher in dogs with giardiasis compared to the healthy group of dogs. Hence, these biomarkers can be considered as an effective and non-invasive method for detecting intestinal mucosal damage in dogs with giardiasis.
CONFLICT OF INTEREST
The authors declare that they have no financial or non-financial conflict of interest regarding authorship and publication of this article.
ACKNOWLEDGMENTS
The research was supported by the Veterinary Faculty at Selçuk University in Konya.
AUTHORS' CONTRIBUTION
MKD, YEE, SSI, NI and ROB did the data curation. MKD, MO, MI and AN wrote the original draft, contributed to conceptualization, design, methodology, writing, and editing of the manuscript.

 10.2478/macvetrev-2025-0024
10.2478/macvetrev-2025-0024

