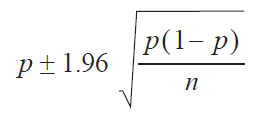Abstract
Swine influenza caused by the influenza A virus significantly affects pig production and pig health due to acute respiratory disease and huge economic losses. Pigs play an essential role in the epidemiology of influenza because they act as a mixing vessel for the formation of potentially pandemic zoonotic strains. The objective of our study was to assess the seroprevalence of swine influenza A viruses (swIAV) in commercial pig farms in Macedonia. A total of 373 blood samples were collected from piglets aged 1 to 4 weeks of sows with different parities from 19 different commercial farrow-to-finish pig farms. For the detection of anti-IAV antibodies, sera samples were analyzed using a competitive ELISA. All farms were seropositive to swIAV. Seropositivity was detected in 258 (69.2%) samples, ranging between 10 and 100% at farm level. The highest seroprevalence was found in piglets from sows in the 5-6 th parity. In contrast, the lowest seropositivity was found in samples from the youngest sows (1-2 nd parity), which indicates that the virus has circulated for a longer period in these farms. Furthermore, large farms with more than 120 sows had a significantly greater percentage of seropositive animals than small farms with less than 120 breeding sows (83% vs. 54%, respectively). In conclusion, our results demonstrated that swIAV circulates endemically in Macedonian commercial farrow-to-finish pig farms, underscoring the need of immunization in preventing infection on these farms.
Keywords: Swine influenza A virus, seroprevalence, pigs, commercial farms, Macedonian
INTRODUCTION
Swine influenza is an acute respiratory disease caused by Swine influenza A virus (swIAV) that significantly affects intensive pig production and pig health worldwide (1, 2). The etiological agent of swine influenza belongs to the Alphainfluenza virus within the Orthomyxoviridae family (3). The virus has three major subtypes (H1N1, H2N1, and H3N2) with genetic components of avian and human influenza viruses resulting in diverse genetic lineages based on geographic region (4, 5, 6). In addition, swIAV has zoonotic potential since it can infect humans (5). Pigs serve as mixing vessels, because different mammalian and avian influenza A viruses (IAVs) can infect them (7). As a result, co-infections in pigs may cause reassortment of genetic segments, producing swIAV strains with novel antigenic characteristics with pandemic potential and the capacity to escape animal and human vaccination immunity (8).
Swine influenza is a highly contagious disease. Infections can result in fever, pneumonia, and weight loss, indirectly causing reproductive problems such as abortion in pregnant sows (
2). Piglet production and feed efficiency are negatively affected by the disease, which results in financial losses for farms as well as detrimental impacts on the economy and food safety (
1). The infection circulates endemically in regions of high pig density (
9,
10), while outbreaks in naive herds are usually followed by high morbidity and low mortality rates (
11). Swine influenza is considered as one of the main viral agents in the development of porcine respiratory disease complex (PRCD), a significant health issue in the world pig industry (
12).
Ascertaining the swIAV seroprevalence in pig farms is particularly helpful in assessing a herd's immune condition, the dynamics of maternally derived antibody (MDA) levels in young piglets, post-vaccination antibody titers, and testing of pigs before moving (
8). According to field studies, persistent infections on pig farms are reported after an acute outbreak and may be related to a combination of the immunological status of the pigs at the time of infection and the transmission of swIAV between batches (
9,
13,
14,
15). Compared to the low frequency of clinical swIAV cases, the majority of reported field cases are subclinical (
16,
17,
18). It was assumed that the recent introduction of swIAV into a pig herd would be associated with a high morbidity rate (
11). Nevertheless, it seems that swIAV is more prevalent and endemic in pigs than previously believed (
19).
Three main swIAV subtypes H1N1, H2N1, and H3N2 have been circulating for years in pig farms across Europe (
4,
8,
18). Over the years, Macedonian pig farmers have imported many pigs from European regions with substantial pig production where the disease is enzootic. In addition, there have been recent reports of the virus circulation in Greece (
20) and Serbia (
21) affecting these two neighboring countries. Due to the absence of a national surveillance system and available vaccines, swIAV may have been introduced and circulating for a long time in Macedonian pig herds. Moreover, 91.2% of the lungs evaluated at the slaughterhouse in a recent study of five commercial pig farms in Macedonia revealed cranio-ventral pulmonary consolidation (
22), which is a lesion that could be associated with swIAV infection.
Based on the previous studies (8, 20, 21, 22), it was hypothesized that swIAV could be highly prevalent and persistent in Macedonian pig farms. The aim of this study was to determine the seroprevalence of swIAV in commercial farrow-to-finish pig farms in Macedonia and to identify variations in the seroprevalence among samples of different sow parity and herd sizes.
MATERIAL AND METHODS
Farms and study design
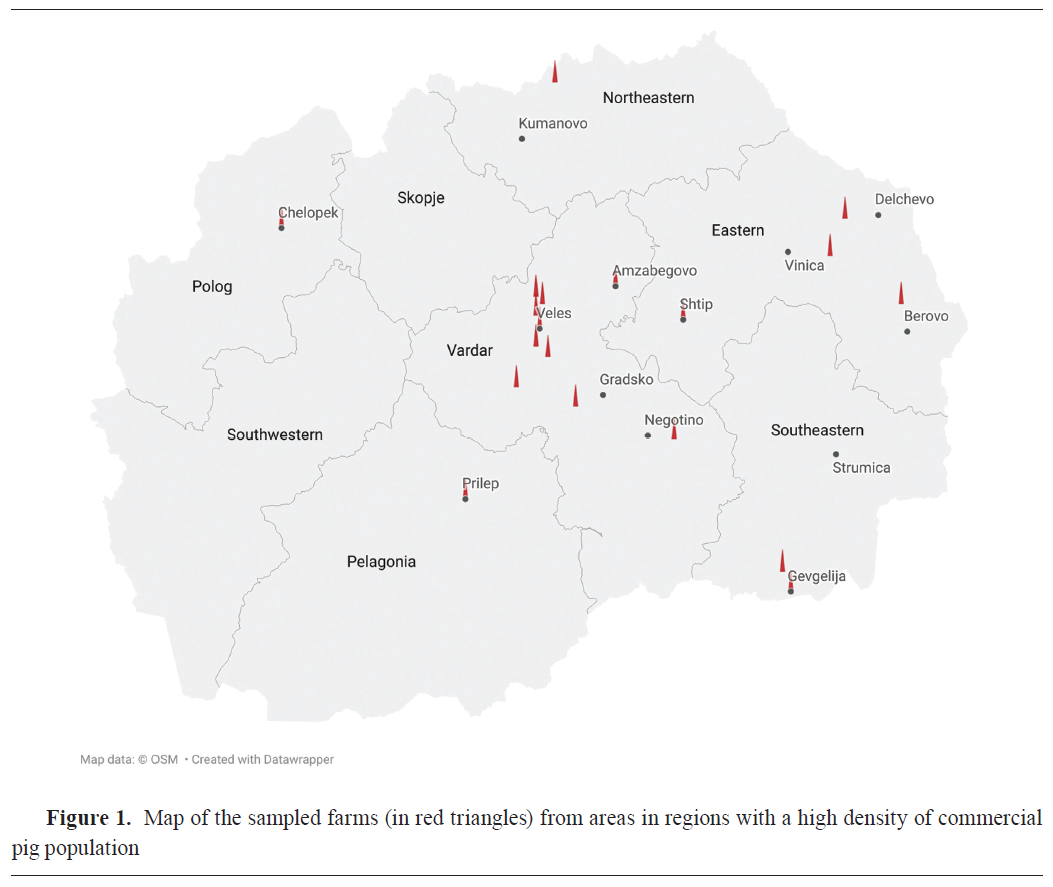
A cross-sectional study was carried out in the period between November 2023 and October 2024 in commercial farrow-to-finish pig farms in the largest commercial pig production (23) and pig density regions in the country such as Veles (Veles, Gradsko, Negotino, Amzabegovo), Eastern (Shtip, Delchevo, Berovo, Vinica), Polog (Chelopek), Northeastern (Kumanovo), Southeastern (Gevgelija), and Pelagonija (Prilep) (Fig. 1).The number and location of commercial farms in the region, the average number of animals per commercial farm, the minimum number of 30 sows kept in the breeding herd, and the farmer's voluntary participation in the study, were the inclusion criteria for the farms in this study. Only one of the farms had conducted vaccinations against swIAV subtypes H1N1, H3N2, and H1N2 (Respiporc® FLU3, Ceva Animal Health, France). The immunization was carried out in gilts at 6 and 3 weeks before the first insemination. All sows are vaccinated twice a year. The sample size was calculated with a minimum 95% probability of detection, diagnostics sensitivity and specificity of 95% and 100%, respectively, and an assumed prevalence of 15% at the animal level and 1% at the herd level. Thus, from 19 commercial farrowing farms (Fig. 1), 373 blood samples were collected from neonatal piglets between the ages of 1 to 4 weeks from sows of different parities. Each animal was restrained and sampled by venipuncture of anterior vena cava using a 19-gauge needle and vacuum blood tubes for serum retrieval. Serum was obtained from all blood samples after centrifugation (10 min at 3,000 g) and were stored at –20 °C for further analysis. Collected and analyzed samples in this study were part of an institutional project (FVMS-IPR-5) funded by the Faculty of Veterinary Medicine in Skopje (Decision no. 0202-359/12 from 31.3.2023).
The samples were grouped according to the sow parity and farm size in order to compare the seroprevalence between large and small farms, and between piglets from sows of different parity. There were four groups of piglet samples according to the sows' parities: 1-2
nd, 3-4
th, 5-6
th, and >6
th (
Table 1). Additionally, the samples were divided into two groups according to the size of the herd: samples from large farms (n=195), with more than 120 sows in the breeding herd, and samples from small farms (n=178), with up to 120 sows (
Table 2). These samples were additionally sub-grouped according to the parity (
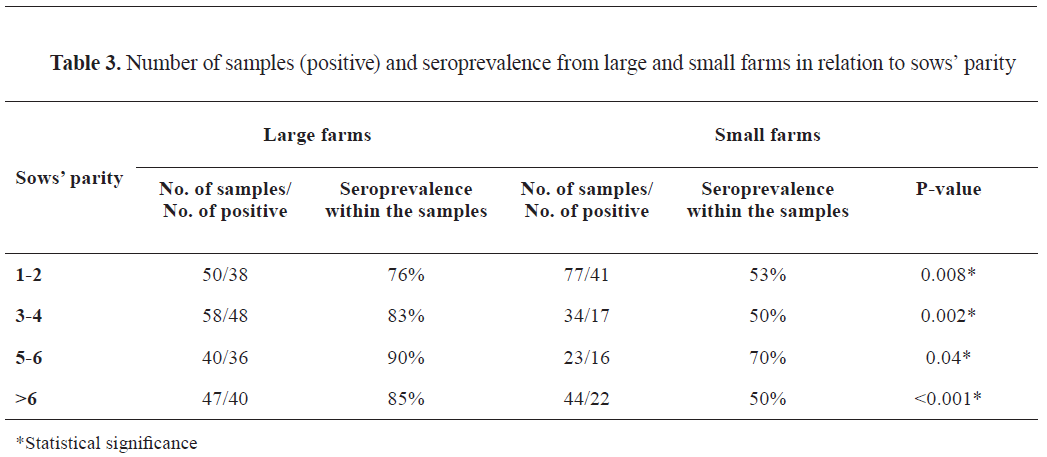
Table 3).
Serology
Serum samples were tested for the presence of anti-IAV antibodies using the AsurDx™ Influenza A Virus (IAV) Antibodies cELISA test kit (BioStone Animal Health, Texas, USA) following the manufacturer's recommendations. The kit detects antibodies against the IAV NP protein of the influenza A virus. Inhibition percentage (IP) was calculated by using optical density data (OD) according to the manufacturer's equation. Inhibition percentage values ≥50% were regarded as positive samples, while IP values <50% were regarded as negative samples.
Data analysis
The binomial confidence intervals of 95% for the overall seroprevalence of swIAV at the animal and farm size levels were calculated by using the following equation:
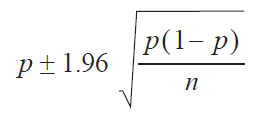
where 'p' is the sample proportion and 'n' is the total number of sampled animals. The statistical analyzes were performed with STATISTICA software (version 8.0; StatSoft, Inc) (24). Variations in the seroprevalence between large and small farms, and between piglets from sows of different ages were compared with the chi-square test. To determine the degree of association between the seropositivity of samples with respect to farm size and various age groups of all samples, the Wald statistic was used to calculate the odds ratios and their 95% CI (25). All results were considered statistically significant at p<0.05.
RESULTS
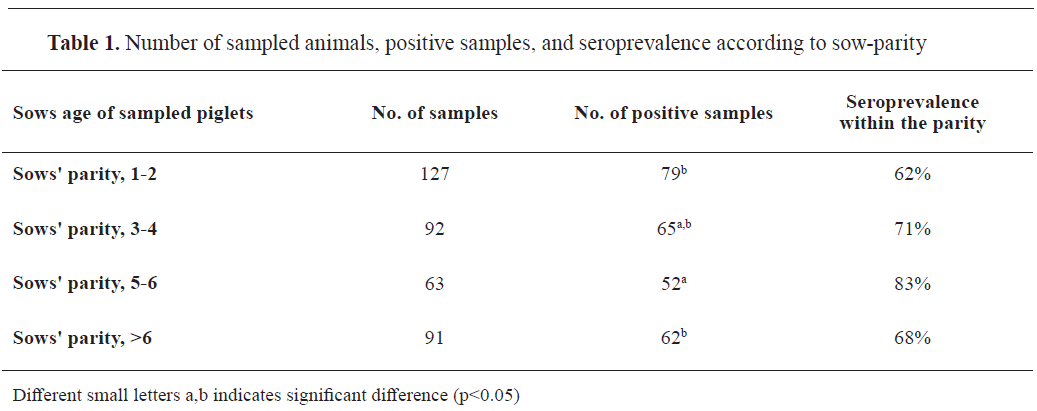
All 19 farms included in the study were seropositive to swIAV with significant difference observed between the herds (χ2) (18)=158.91, p<0.001). Seroprevalence within-farm ranged from 10 to 100%. Out of 373 sampled animals, 258 were seropositive (69.2%; 95% CI: 64.5-73.5%). Samples from piglets of sows in 5-6
th parity had the highest seroprevalence (83%) compared to the samples from other sow groups (
Table 1). Piglet samples from sows in 5-6
th parity were 2.87 times more likely to be seropositive (p=0.003, 95% CI: 1.38; 6.06) compared to the samples from piglets of sows in 1-2
nd parity. Moreover, samples from piglets of sows in 5-6
th parity were 2.21 times more likely to be positive (p=0.03, 95% CI: 1.002; 4.85) than samples from piglets of sows in >6
th parity (
Table 1).
Significantly (p<0.001) higher number (162/195, 83.0%; 95% CI: 77.3-88.3%) of positive samples were recorded in large farms compared to small farms (96/178, 53.9%; 95% CI: 46.6-61.2%) (Table 2). Large farm samples were approximately 4 times more likely to be positive compared to small farm samples (OR=4.17, 95% CI: 2.60; 6.68). Seroprevalence to swIAV had high variability among herds on small farms. It ranged between 44% and 95% on large farms (Table 2).
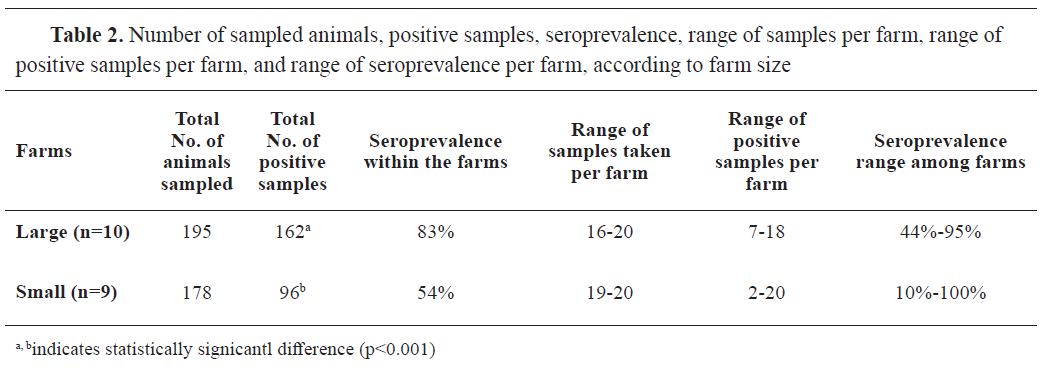
Table 3 summarizes the results of seropositivity in large and small farms in relation to sows' parity (age category). Large farm samples in all age categories had a significantly higher number of positive findings compared to the small farms' samples. Specifically, the greatest difference in seropositivity was found between the oldest sows' group in large farms (>6th parity) ((χ2 (1)=12.90, p<0.001) and the same sow age group from small farms (85% vs. 50%, respectively). The lowest difference in the seroprevalence between large and small farms was observed in samples from piglets of sows' 5-6th parity (90% vs. 70%, respectively).
DISCUSSION
The current study assessed the seroprevalence of swIAV infections in Macedonian commercial pig farms by testing 19 farrow-to-finish pig herds for a presence of swIAV antibodies. To the best knowledge of the authors, this is the first study assessing the seroprevalence of swIAV in Macedonian commercial pig farms. Suckling piglets between 1 and 4 weeks of age from sows of different parity, were sampled in order to identify the presence of specific swIAV antibodies. Colostrum consumption provides specific swIAV antibodies which are detectable between 10 and 14 weeks of age (
26,
27,
28). These MDA reflect the immune status of the sow which in turn is attained by vaccination or infection (
8). Eighteen out of nineteen farms in the current study had non-vaccinated sows against influenza. Hence, the observed high seroprevalence (69%), with at least two seropositive animals per farm, suggests that existing antibodies were most likely developed due to natural exposure to influenza A virus. The existing antibodies in the vaccinated sows and the high seroprevalence (85%) in the single farm, could not be clearly explained by past infection or immunization. Pigs may be exposed to IAV from birds, humans, or other pigs introduced in the farms and can get infected with more than one strain at the same time (
8,
15). Similar findings were reported by Ferreira et al. (
28) where the swIAV seroprevalence was 68% in 11 pig nursery farms in the Brazilian state of Santa Catarina. Another study in Brazil reported seropositivity to swIAV in all farms with roughly 64% of the sows being seropositive to the virus (
29). According to Simon- Grife et al. (
16), 93.9% of Spanish pig farms revealed seropositivity to swIAV, with an overall animal-level seroprevalence of 62.3%, and with significantly higher prevalence in sows (89.9%) than in finishing pigs (53.1%). On the contrary, Papatsiros et al. (
20) found a lower mean seroprevalence of 35% in Greek farrow-to-finish pig farms. Jungic et al. (
30), reported lower seroprevalence (30.3%) in Croatian pig farms. Greater proportions of seropositive pigs in Macedonian farms might be related to the frequent import of live pigs from Denmark and Netherlands where continuous circulation of swIAV has been noted (
4,
8). Simon et al. (
4) found that the Netherlands and Denmark were among the top EU countries where swIAV had spread, affecting 42% and 48% of tested herds, respectively. According to the seroprevalence found in our study, a significant proportion of sows transferred swIAV-specific MDA to their young piglets. These antibodies can protect suckling and weaner pigs from clinical manifestation of swIAV but they are unable to prevent the infection due to the existence of multiple virus subtypes on the farm (
3,
15). Piglets from 5-6
th parity sows had the highest seroprevalence to swIAV, whereas lowest prevalence was observed in piglets for 1-2
nd parity sows. Piglets from 5-6
th parity sows had threefold higher probability to be seropositive compared to the piglets from the youngest sows. This finding in the current study was consistent with other reports where higher parity sows were also noted to have a greater likelihood of being seropositive to swIAV (
29,
31,
32). The higher seroprevalence in piglets from the older sows (≥3 parity) suggests that swIAV is continuously circulating in the farms under continuous monitoring and that older animals experience recurrent infections because of their extended exposure to the virus. Another possible explanation for the lower seroprevalence in piglets from younger sows (gilts) is that they acquire lower colostrum immunity compared to the piglets from older sows (
33). The current study has also demonstrated that piglets from 5-6
th parity sows had higher chance of being seropositive than piglets from the oldest sows’ group (>6 parity). The lower seroprevalence in the piglets from older sows may be explained with the inadequate consumption and low-quality colostrum which results in lower serum swIAV-specific antibodies concentration. Older sows produce lower colostrum (
34), are more likely to be obese, develop limb injuries, and have lower physical activity compared to younger sows (
35).
Piglets from large farms had a much higher swIAV seroprevalence compared to piglets from small farms, regardless of the sows’ parity. This finding was similar to several studies which reported greater risk for swIAV infection in large herds with high pig density (
16,
31,
32,
36,
37) due to the higher virus circulation and evolving which can result in spill over to the human population. Larger herds usually have more frequent direct and indirect contact with other animals, workers, visitors, and vehicles. The frequent human-pig interaction on these farms (
38) is raising the likelihood of swIAV infections (reverse zoonosis). There have been numerous reports from different countries where IAV was transmitted backward from people (workers, farmers, and visitors) to pigs (
39). The potential for human-to-pig transmission is well shown by the swIAV seroprevalence rate in pigs and pandemic IAV infection wave in humans. Due to the higher IAV exposure, pig farm workers have higher antibody titers, which can be used for monitoring influenza dynamics (
40). One of the risk factors for higher swIAV seroprevalence in pig herds was higher gilt replacement rate (
16,
41,
42,
43).
Serafini Poeta Silva et al. (
29) reported that farms replacing gilts by introducing new animals had a much greater swIAV seroprevalence than farms that produced their own gilts. According to these authors, providing acclimatization units for newly introduced animals is one of the strategies to prevent introduction of influenza virus in the farms. According to Duffy et al. (
42), replacement gilts are introduced more frequently in large than in small herds, which could explain the higher seroprevalence of swIAV on large farms in this study. As a result, the regular introduction of new animals increases the risk of introducing susceptible (naïve) and infected animals in the herd enabling the virus to spread. The high swIAV seroprevalence in the current research could be explained by the type of farms that were sampled. Farrow-to-finish herds with continuous pig flow have higher seroprevalence and continuous circulation of swIAV in pig herds (
13,
31,
36,
43). It is challenging to implement strict all-in/all-out procedures for nursery units at these farms, which are often carried out on a pen base. In this regard, high swIAV circulation is highly correlated with the practice of mixing piglets from various litters which may have previously infected and recovered individuals (
43). In general, the implementation of proper farm biosecurity practices and vaccination can prevent transmission and can decrease the incidence of swIAV in pig herds (
28,
36,
44).
Numerous factors, such as farming practices, population dynamics, herd immune status, and co-circulation of different subtypes, affect the epidemiology of swIAV, even though suckling piglets play a major role in the transmission and maintenance of the disease in endemically infected pig farms (27). To gain a better knowledge of swIAV dynamics in Macedonian commercial pig farms, more research on swIAV epidemiology is required. CONCLUSION
The high seroprevalence in the current study indicates that swIAV circulates endemically in Macedonian commercial farrow-to-finish pig farms. It is evident that swIAV circulated over a longer period of time in these farms since piglets from older sows (5-6th parity) showed a greater seroprevalence than piglets from youngest sows (1-2nd parity). Large farms had higher seroprevalence than small farms, which suggests that the virus is more widespread in large breeding herds. Our research emphasizes the importance of serological testing in commercial farrowing pig farms and the necessity of implementing vaccination programs. However, more research on the molecular epidemiology of swIAV is crucial to better understand genetic diversity and to adequately apply preventive and control measures in commercial pig farms. CONFLICT OF INTEREST
The authors declare that they have no financial or non-financial conflict of interest regarding authorship and publication of this article.
ACKNOWLEDGMENTS
Pig farms are acknowledged for their contribution to this study. The authors would like to thank the colleagues from the Laboratory of Serology from the Faculty of Veterinary Medicine in Skopje for their technical support with the ELISA testing. Great acknowledgment to Dr. Kathrin Lillie-Jaschinski from Ceva Sante Animale- Germany for her expert advice regarding sampling approach. This study was founded by the Faculty of Veterinary Medicine–Skopje within the internal project FVMS-IPR-5 (Decision no. 0202-359/12).
AUTHORS' CONTRIBUTION
BA developed the study design and concept, collected samples, and wrote a manuscript. MKJ revised the manuscript and analyzed the obtained data. AJ was involved in sample collection and contributed to manuscript writing. JB contributed in writing the manuscript and gave critical review for important intellectual content. AD was involved in data analysis and interpreted ELISA results. All authors have revised and approved the final version of the manuscript.

 10.2478/macvetrev-2025-0025
10.2478/macvetrev-2025-0025