INTRODUCTION
Polycystic ovarian syndrome (PCOS) is a complicated self-perpetuating endocrinopathy with worldwide occurrence ranging from 5-20% among females of reproductive age (
1). PCOS encompasses the complex interplay of neuroendocrine, reproductive and metabolic disorders driven by a variety of environmental and hereditary variables (
2). Hyperandrogenism, menstrual irregularity and polycystic ovarian morphology are most persistent characteristics of PCOS, which eventually leads to anovulatory infertility (
3). Prolonged hyperandrogenism causes alteration in gonadotropin secretion, disruption of the hypothalamic-pituitary-gonadal axis, abrupt luteinization of granulosa cells and aberration of ovarian steroidogenesis (
4). Atypical expression of androgen-producing steroidogenic enzyme 3βHSD is associated with consistent development of cystic follicles (
5). Furthermore, improper functioning of the major female sex hormone estradiol via estradiol receptors (ERα and ERβ) also contribute to PCOS by affecting egg maturation and ovulation (
6). In addition, excess androgen also disrupts metabolic homeostasis by developing central adiposity, dyslipidemia, and hyperglycemia with decreased insulin sensitivity (
3). Hyperglycemic condition stimulates ROS generation from the leukocytes by eliciting a pro-oxidative response in PCOS (
7). The etiology of PCOS in the context of oxidative stress may entail cellular organelle dysfunction as documented from the report of metabolic and molecular alterations (
8). Nevertheless, it is undefined if oxidative stress contributes directly in the establishment of PCOS or if it only develops as a secondary ailment due to hyperglycemia and insulin resistance.
Altogether, the complex combination of all these factors makes PCOS a challenging condition for executing proper diagnosis and treatment. Modern therapeutic strategies frequently involve a combined medication therapy including anti-androgenic drugs, metformin and oral contraceptive pills for the treatment of PCOS. Metformin is effective to treat metabolic disturbances in obese PCOS women by normalizing the glycemic homeostasis (
9). Antiandrogenic drugs are often used in treatment of clinical hyperandrogenism such as hirsutism in PCOS (
10). Combined oral contraceptives are mainly used in the improvement of menstrual irregularities and provide protection to endometrium in PCOS (
11). The presently available medical interventions, have merely alleviating effects and usually have severe side effects (
12). This has boosted the interest in traditional effective herbal remedies which are safer and non-invasive in the treatment of PCOS. Ayurvedic therapies for PCOS strive to maintain optimal
dosha (energies) balance, notably
Vata (biological air or movement energy) and
Kapha (biological water or mucus), through dietary adjustments, herbal medications, lifestyle changes, and stress management (
13). Implementing herbal remedies in the regular diet has become a new trend for managing PCOS (
14). Shatavari (
Asparagus racemosus) and nirgundi (
Vitex negundo) are famous traditional Ayurvedic herbs with broad range of therapeutic advantages (
15,
16). Ayurveda describes Shatavari as ‘the Queen of herbs’ with revitalizing properties that limits women’s reproductive health disorders by soothing
Vata and
Pitta (metabolic energy) doshas (
15,
17). On the other hand,
Vitex negundo is well known as Nirgundu, Punjgusht, Sephali, or Sambhalu with a balancing impact on
Vata and
kapha doshas (
18,
19). The roots of
A. racemosus is a multifunctional hormone-balancing feminine tonic that promotes fertility by nourishing reproductive organs. Previous clinical study also suggested ayurvedic formulation including shatavari and other herbs which were successfully used for treatment of subfertility in PCOS women (
20). However, the seeds of
V. negundo counteracts hyperandrogenism and thereby maintains the normal follicular advancement, minimizing PCOS symptoms (
21). Several studies have pointed out that both herbs possess antioxidant, hypolipidemic, immunomodulatory, and anti-inflammatory properties without producing undesired side effects (
16,
22). Although,
A. racemosus and
V. negundo have been extensively studied for their medicinal properties, there are scarce evidence on their effect in the treatment of PCOS. Hence, this study aimed to assess the therapeutic effect of
A. racemosus (roots) and
V. negundo (seeds) on estrous cycle patterns, estradiol level and its receptors expression, steroidogenesis, folliculogenesis, metabolic parameters, and oxidative/antioxidative status in Wistar rats with PCOS. Additionally, the study focused to elucidate the pathophysiological mechanisms of PCOS through assessment of these effects.
MATERIAL AND METHODS
Chemicals
Letrozole tablets were obtained from Sun Pharmaceutical Ltd, Bengaluru, India. All the consumables were provided from Hi Media (India), and Merck (India).
Aqueous extraction of A. racemosus roots and V. negundo seeds
Roots of
A. racemosus and seeds of
Vitex negundo were collected from the local Ayurvedic Pharmaceutical Shop (Midnapore, West Bengal) and grounded into powder form. Each set of powdered herbs of 100 g was dissolved into 1000 mL of distilled water and continuously stirred for 2 days before filtration. The filtrates were then concentrated in a rotary evaporator. Collected concentrates were air-dried and stored for the experiment. The total yield of the aqueous extracts of
A. racemosus and
V. negundo were 17.23 g and 13.73 g, respectively. Roots and seeds of
A. racemosus and
V. negundo were used according previous reports (
21,
23).
S
election of specific dosages
A. racemosus and
V. negundo were administered at a dose of 250 mg/kg BW according to the findings of a previous
in vitro screening (data not shown) and published reports (
21,
23).
Animal selection and care
Female albino rats (n=24), with 100-120 g body weight and 6-8 weeks old, were acclimatized for 8 days at 25 °C and 50-70% humidity. The animals were procured from an authorized animal provider (M/S Chakraborty Enterprise; Regd no. 1443/PO/ Bt/s/11/CPCSEA; India). All the rats were kept in polycarbonate cages with adequate access to food and water ad libitum.
Ethical approval
The Animal Ethics Committee of Vidyasagar University approved the experiment with ethical clearance number VU/IAEC/CPCSEA/19/7/2022 dt.22.11.2022 (
A. racemosus) and VU/IAEC/ CPCSEA/17/7/2022 dt.22.11.2022 (
V. negundo). The experiment was carried out in compliance with the norms specified by the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), India. This study also followed the EU Directive guidelines (2010/63/EU) for the protection and care of laboratory animals and experimental protocols used for scientific purposes.
PCOS induction and experimental design
Letrozole (1.0 mg/kg BW) was diluted in 0.5% carboxymethyl cellulose (CMC) and administered orally for 21 consecutive days to develop PCOS (20). Total twenty-four Wistar rats were divided into the following four groups:
- Control Group: received 0.5% CMC orally for 21 days;
- PCOS Group: letrozole (1.0 mg/kg BW) was suspended in 0.5% CMC and administered orally to rats for the first 21 days (24, 25);
- ARA Group: oral administration of letrozole (1.0 mg/kg BW) for 1st 21 days + post administration of ARA (A. racemosus roots aqueous extract; 250 mg/kg) via oral route for the next 21 days (23);
- VNA Group: oral administration of letrozole (1mg/kg BW) for 1st 21 days + post administration of VNA (V. negundo seeds aqueous extract; 250 mg/kg) orally for the next 21 days (21).
The experiment was conducted for 42 days. Rats were closely monitored regularly for changes in body weight, estrous cycle, and other discomfort signs. On day 43, all rats were sacrificed using anesthetic Ketamine/HCl (80 mg/kg BW, intraperitoneal injection) and xylazine (8 mg/kg BW, intraperitoneal injection) following the guidelines of CPCSEA, India. Serum and organs were procured and stored at -20 °C for further analysis.
Vaginal smear observation
Vaginal cytology studies were performed on regular basis throughout the whole investigation. A sterile dropper filled with 100 μL of normal saline was carefully inserted into the vaginal region of the rat for the collection of vaginal fluid. Collected vaginal fluid was transferred on glass slides and was air-dried. Slides were then stained with Leishman stain and observed under a light microscope.
Body weight and organs weight measurement
The body weight of all animals was monitored regularly throughout the animal treatment. The final weight was recorded according to the standard protocol. Reproductive organs weight was also measured after sacrificing.
Serum analysis
The serum was collected from the blood following centrifugation at 2,500 rpm for 6 min. Serum glucose, triglycerides, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels in serum were measured using kit assay procedures according to manufacturer specifications.
ELISA of estradiol, estradiol receptor (ESR1), and steroidogenic enzyme 3-beta hydroxysteroid dehydrogenase (3βHSD)
ELISA kits were used to assess serum estradiol,
ESR1, and 3βHSD (ovarian tissue specific) in compliance with the manufacturer’s recommendations. The reading was taken spectrophotometrically at 450 nm.
Spectrophotometric studies of superoxide dismutase (SOD) and catalase
Ovarian tissue (50 mg/mL) was homogenized in ice-cold lysis buffer (pH 8.4) and then centrifuged at 10,000 g for 15 min at 4 °C. The recovered supernatant (50 μL) was mixed with 10 mM pyrogallol and 50 mM Tris-HCl (pH 8.4), and wavelength was set at 420 nm to measure the absorbance change for SOD. The catalase was measured with the remaining part of the supernatant which was mixed with H
2O
2 solution. The change in absorbance was noted at 240 nm.
Native gel expression of SOD and catalase
The expression study of SOD and catalase was performed by using 12% and 8% native gels, respectively. Ovarian tissue (50 mg) was homogenized with chilled phosphate buffer solution (PBS; 1.0 mol/L, pH 7.4) and centrifuged at 10,000 g for 15 min at 4 °C. The tissue protein extract (50 μg) was further applied on 12% and 8% native PAGE.
Following electrophoresis, the 12% gel was incubated for 20 min in dark with 28 mM TEMED (tetramethylethylenediamine), 2.3 mM NBT (nitroblue tetrazolium), and 28 mM riboflavin to observe SOD expression (
26).
Similarly, the 8% gel was incubated in 0.003% H
2O
2 solution before being exposed to 2% potassium ferricyanide and 2% ferric chloride solutions to generate achromatic catalase band (
26).
Densitometry of the above achromatic band of electrozymogram was determined by the Image J software 1.54, National Institutes of Health, USA.
Histological study
The ovaries were promptly obtained and stored in formalin upon sacrifice. The tissue was then fixed with paraformaldehyde and dehydrated with increasing concentration of ethanol. Following washing with xylene, ovarian tissues were fixed in paraffin and were serially sectioned with 5 μm thickness. Staining was performed with hematoxylin and eosin. The slides were viewed under light microscope.
Statistical analysis
ANOVA followed by the post hoc Tukey’s test was performed using SPSS 16.0 software for the determination of statistical significance between the groups.
RESULTS
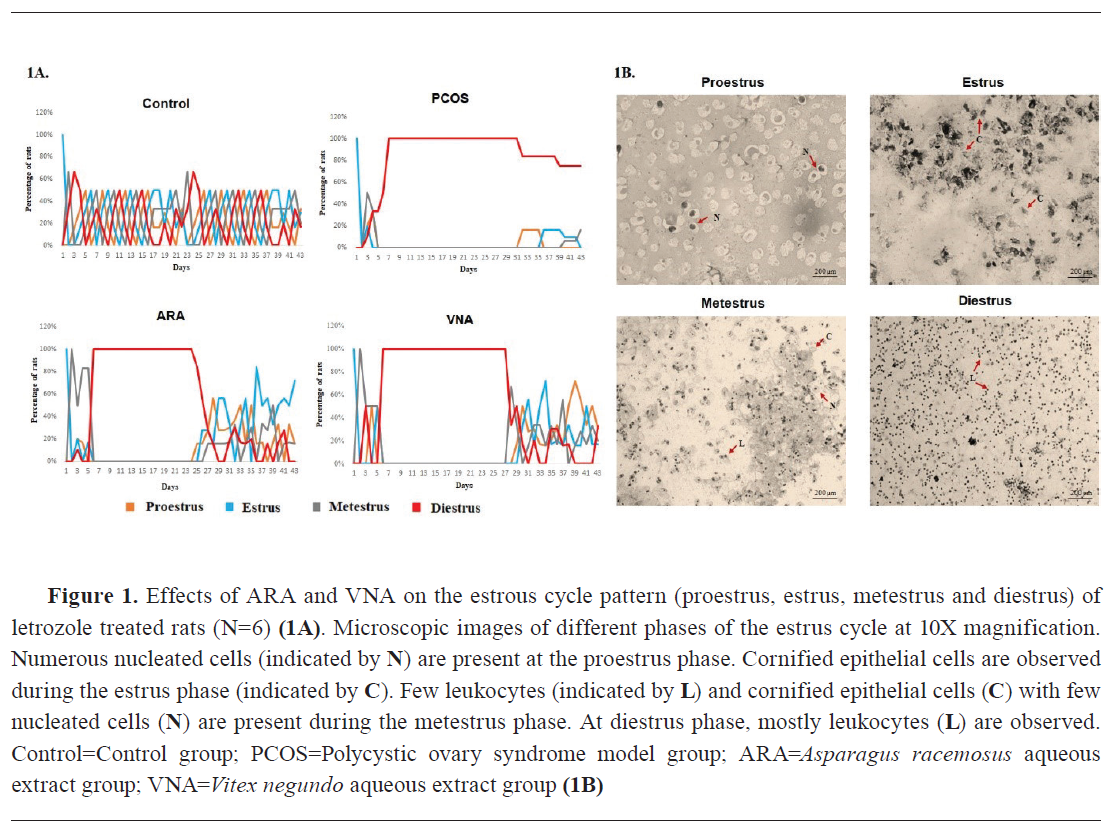
Effect on estrous cycle pattern
Letrozole-induced PCOS group showed alteration in estrous cycle followed by continuous diestrus phase. The vehicle-treated control group had typical estrous patterns. ARA and VNA groups showed longer estrus phase and decreased duration of the diestrus phase compared with the PCOS group (
Fig. 1A and 1B).
 Effect on body weight and organosomatic indices
Effect on body weight and organosomatic indices
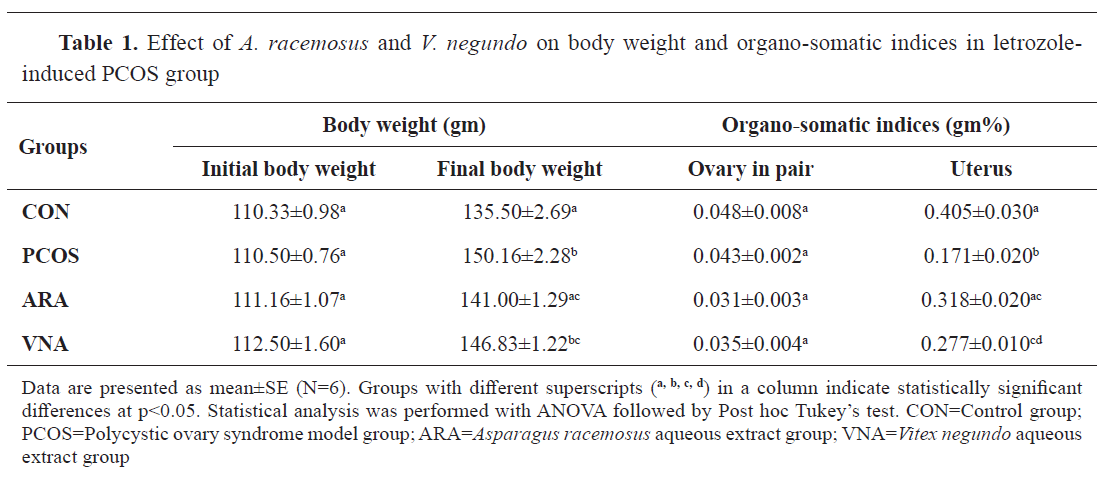
A significantly higher body weight was observed in PCOS rats compared to the control group (
Table 1). ARA and VNA supplementation had significantly lower body weight in PCOS-induced rats (
Table 1). No significant change in the ovarian weight was observed compared to the control. Uterine weight was significantly lower in PCOS rats compared to the control. Supplementation with ARA and VNA significantly (
Table 1,
Fig. 2) attenuated the uterine weight of PCOS rats.
 Serum glucose and lipid profile
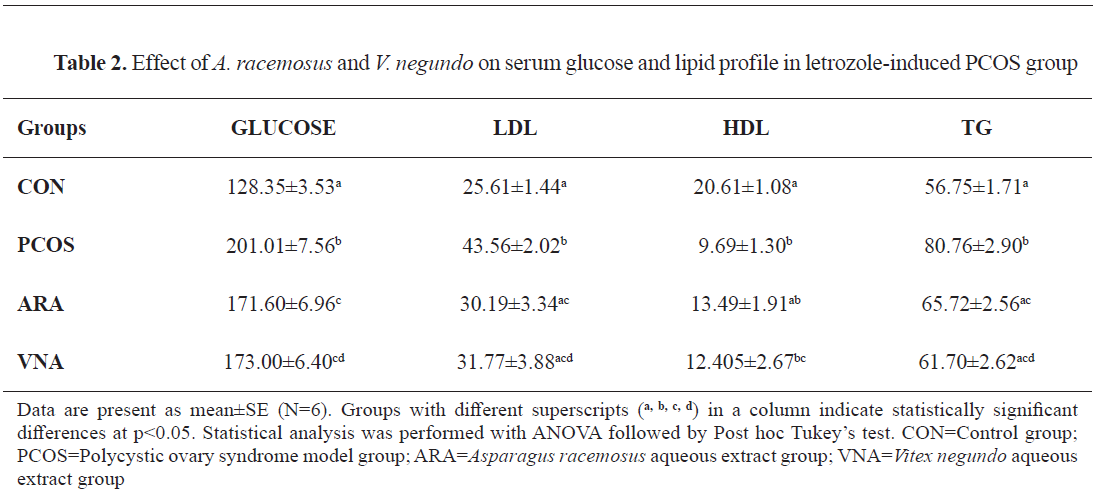
Serum glucose and lipid profile
Significantly higher levels of blood glucose and dyslipidemia were observed in PCOS group than that of the control group. A significantly lower serum glucose, LDL, and triglyceride were observed in the ARA and VNA post-treated groups (
Table 2).
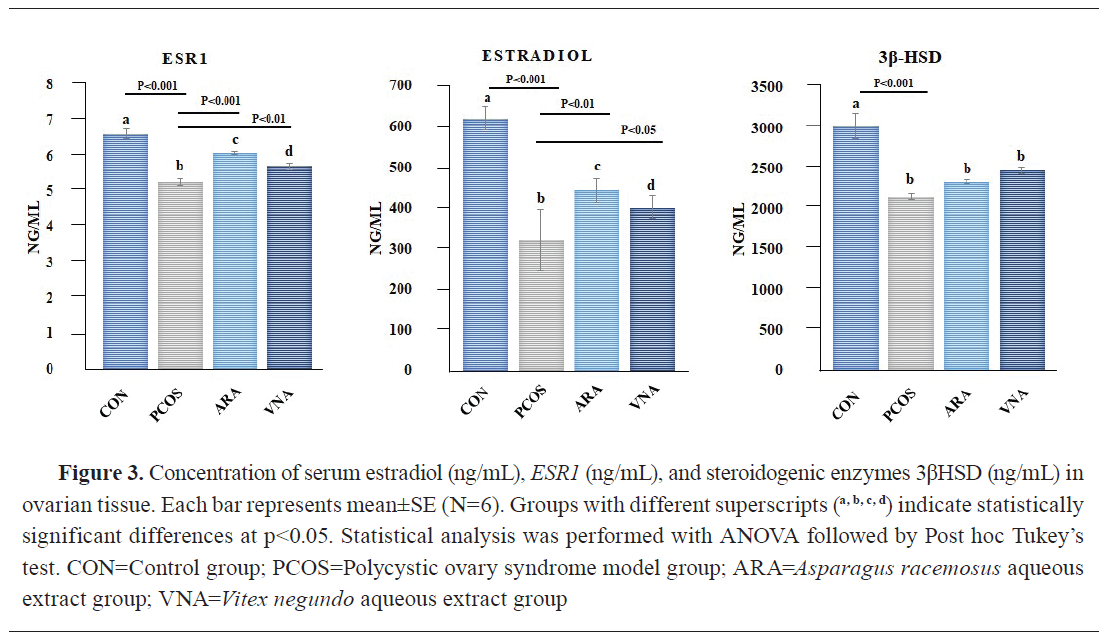
 Effect on estradiol, ESR1, and 3βHSD
Effect on estradiol, ESR1, and 3βHSD
A significant suppression of estradiol level and weak signaling of
ESR1 were observed in the PCOS group compared to the control. Decreased concentration of the steroidogenic enzyme 3βHSD was also observed in the PCOS group. Post-treatment of ARA and VNA significantly upregulated the action of estradiol and
ESR1, whereas a non-significant increase in 3βHSD was noted following treatment (
Fig. 3).
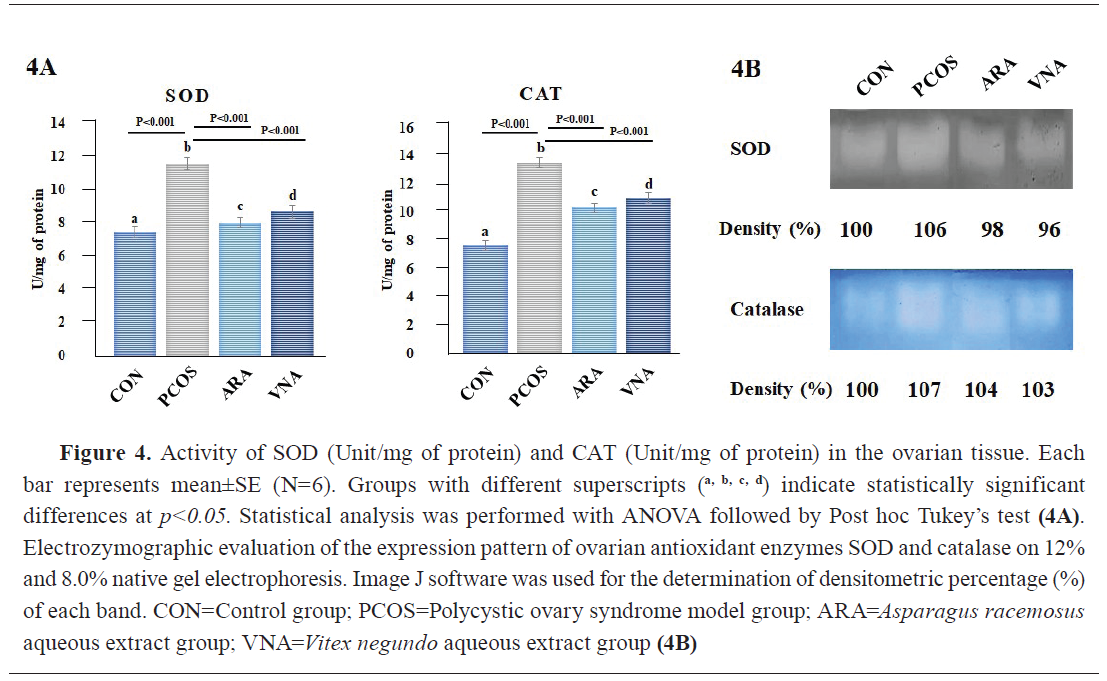
 Antioxidant status
Antioxidant status
Pyrogallol autoxidation and H
2O
2 test revealed significantly higher activity of SOD and catalase in PCOS compared to the other groups (
Fig. 4A). The ARA and VNA groups had non-significantly different antioxidant enzyme activity compared to the control (
Fig. 4A). Native gel expression showed higher band density of these antioxidant enzymes in the PCOS group and lower density in ARA and VNA groups (
Fig. 4B).
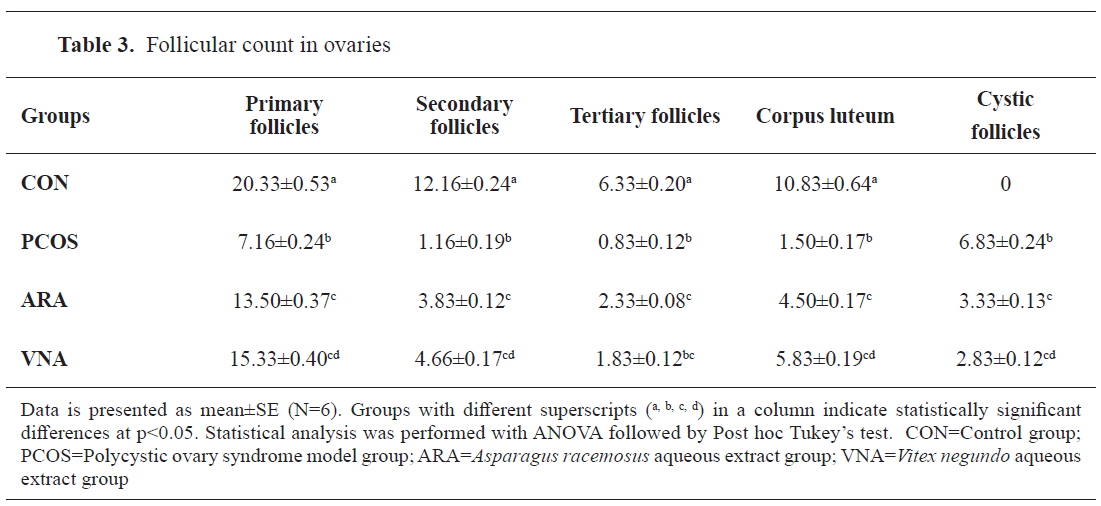
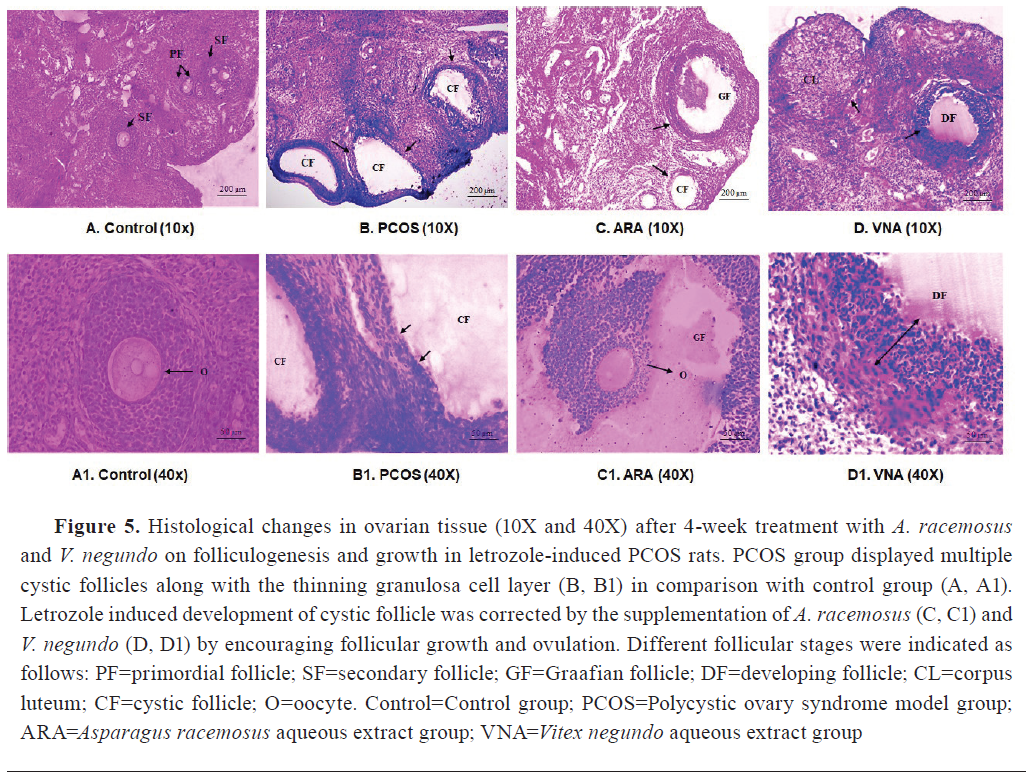
 Histological analysis of ovary
Histological analysis of ovary
Light-microscopic images (10x and 40x) of ovary sections of the letrozole treated group (
Fig. 5,
Table 3) displayed multiple cystic follicles characterized with diminished granulosa cell layer and enhanced follicular antrum. The ARA and VNA groups had lower number of observable cystic follicles compared with PCOS rats (
Fig. 5,
Table 3).
 DISCUSSION
DISCUSSION
Letrozole is well documented to induce
in vivo PCOS by interrupting the estrogen conversion from testosterone and causing excessive androgen accumulation in the ovaries, leading to endocrine imbalance (
24). Hormonal alterations negatively perturbed the estrous cyclicity in the letrozole-treated rats. A continuous diestrus phase was observed in the PCOS group, as reflected by the predominant presence of leukocytes in the vaginal smear (
Fig. 1). Administration of ARA and VNA for 21 days following the PCOS development tended towards a steadiness of normal estrous cyclicity by replacing the acyclic estrous pattern. This was confirmed from the exclusive presence of nucleated and non-nucleated cornified epithelium cells (
Fig. 1).
Hyperandrogenism-mediated feedback signal to the pituitary gland leads to increased LH and decreased FSH output (
27). Normally, diffusion of androgen to granulosa cells from theca cells acts as the substrate for estradiol formation in response to FSH (
28). Alteration in LH:FSH ratio may further cause progression and persistence of sex steroid alteration. The present investigation observed aberrant estradiol and
ESR1 activity in ovarian tissue, indicating improper estradiol signaling in letrozole-induced PCOS group (
Fig. 3). Estradiol regulates ovarian follicular growth and maturation via
ESR1 and
ESR2 receptors (
29). Additionally, this analysis found greater evidence of ovarian disturbances caused by uterine horn shrinkage (
Fig. 2). A prior study confirmed the dose-dependent suppression of uterine weight in letrozole-induced PCOS (
30). Dose-dependent uterine suppression may be due to reduced estradiol concentration, as circulating estradiol is critical for uterine growth (
31). The phytoestrogenic actions of ARA and VNA significantly modified the endocrinal imbalance by enhancing circulatory estradiol and
ESR1 signaling (
Fig. 3). In response to the structural and functional similarity with the mammalian 17β-estradiol, the binding of phytoestrogens to the estrogen receptors could exert an agonist or antagonistic effect on estrogen-responsive gene products (
32). Administration of
A. racemosus demonstrated estrogenic effect on genital organs and mammary glands in pregnant rats (
33). At the same time,
V. negundo has also been reported to enhance the estradiol level in letrozole-induced PCOS (
21). Our results also confirmed the hormone modulatory properties of
A. racemosus and
V. negundo, which in turn helps in modifying the estradiol and ER-mediated signaling in PCOS.
Production of the thecal steroidogenic enzymes is completely reliant on LH (
34). Previous studies showed that cystic follicles express a higher amount of 3βHSD compared to the normal follicles (
35). The presence of higher 3βHSD activity in ovarian granulosa cells of cystic follicles may provide additional precursors in the theca cells for the conversion of DHEA, which further leads to greater androgen production (
35). Reduction in steroidogenic enzyme 3βHSD in the letrozole-treated ovaries is another exceptional result observed in the present study (
Fig. 3). Although, 3βHSD is an important enzyme for androgen production, it is also essential for progesterone biosynthesis (36). A decrease in 3βHSD may affect the synthesis of progesterone, resulting in a drop in FSH and an alteration in the LH:FSH ratio in the gonadal axis (
31). Post-administration of ARA and VNA both exhibited minimal and non-significant enhancement in the concentration of ovarian 3βHSD (
Fig. 3), which signifies plausible uninterrupted progesterone biosynthesis.
In addition to the reproductive abnormalities, many women with PCOS also experience various metabolic issues like dyslipidemia, hyperglycemia, central adiposity, and insulin resistance, raising the risk of type 2 diabetes and cardiovascular disease (
37). Hyperandrogenism is preferentially linked to intra-abdominal fat deposition and enhanced numbers of tiny subcutaneous abdominal adipocytes, which may limit subcutaneous adipose storage and thereby encourages metabolic dysfunction (
38). The present investigation demonstrated that the letrozole-induced PCOS model exhibits several metabolic abnormalities, observed in PCOS women. Letrozole treated rats showed a significant increase in the body weight (
Table 1). The letrozole group also displayed an enhancement of serum glucose along with the alteration of lipid profile (
Table 2). Supplementation of
A. racemosus successfully reduced the body weight significantly, whereas
V. negundo showed non-significant effect. Both herbs exhibited potential hypoglycemic efficacy by controlling the blood glucose level against PCOS (
Table 2). Enhanced levels of glycerol, cholesterol, and LDL have been noticed in the follicular fluids of PCOS patients, which affect the oocyte environment (
39). ARA and VNA reversed this dyslipidemic condition by significantly reducing the triglycerides and LDL levels, but no significant alteration was detected for HDL (
Table 2).
The intraovarian folliculogenesis process is also regulated by oxidative metabolism (
8). Excessive ROS production affects meiosis II progression, abolishes gonadotropin secretion, DNA damage, and inhibits ATP production (
8). PCOS has been linked with increased oxidative stress, which ultimately leads to intra-ovarian disturbance by impairing oocyte maturation and luteal progression (
40). Earlier studies also established that letrozole-induced PCOS rats showed a suppression of antioxidant enzymes (
41). Surprisingly, our present investigation noticed the increased activity of SOD and catalase in the letrozole-induced PCOS group (
Fig. 4A and 4B). The increased activity of antioxidant enzymes reflected activation of intrinsic cellular defense mechanism to compensate the elevated oxidative stress in PCOS group. Since, letrozole was given for only the initial 21 days of the experiment, it was unable to preserve a significant level of oxidative stress during letrozole withdrawal for the next 21 days. The above instances may be due to the reversible mode of action where letrozole cessation immediately transits towards rapid recovery to adjust the normal homeostasis that results in reduced oxidative stress in the PCOS group. Despite the reduced oxidative stress, letrozole successfully developed polycystic ovaries as confirmed from the ovarian histoarchitecture (
Fig. 5). Administration of ARA and VNA immediately after letrozole cessation showed a sharp reduction in antioxidant enzyme activity and its subsequent expression when compared to the PCOS group. However, the antioxidant activities (SOD and catalase) in both groups returned to baseline levels similar to vehicle-treated control group (
Fig. 4A and 4B). From this specific angle, this result implied that the ARA and VNA effectively normalized the redox status without overstimulating the cellular antioxidant defense system. These observed findings indicated that the oxidative stress reduction might not be the primary therapeutic pathway involved in restoration of PCOS. Despite the contradiction in the status of antioxidative enzymes,
A. racemosus and
V. negundo have successfully corrected the reproductive as well as metabolic dysfunction in PCOS. These observed findings imply that the action of ARA and VNA may be target-specific involving hormonal regulation and improved metabolic homeostasis regardless of oxidative stress.
Histology of the letrozole-treated ovaries showed the presence of multiple cystic follicles with thinning of ovarian granulosa cells (
Fig. 5).
ARA and VNA resulted in successful elimination of PCOS-associated symptoms, thickened granulosa cell layer, and maintenance of proper interaction between theca and granulosa cells (
Fig. 5). Supplementation with
A. racemosus promotes ovulation which was confirmed by the presence of graafian follicles, whereas
V. negundo maintains follicular progression and maturation by dissolving the cystic follicles (
Table 3,
Fig. 5).
CONCLUSION
ARA and VNA successfully improved the metabolic and reproductive status in PCOS rats. It reduced cystic follicle occurrence and maintained usual folliculogenesis in an oxidative stress independent pathway. Additionally, the phytoestrogenic action of these herbs improved
ESR1 signaling by restoring the levels of estrogen and its receptors. This aided in synchronizing the estrous cycle during PCOS. However, more detailed investigations on hormonal signaling pathways and hypothalamic-pituitary-adrenal (HPA) axis are imperative for the appropriate explanation of the mechanism of action of these herbal drugs. Moreover, exploring multi dose dependent studies of these herbs in higher animal models and human trials is also necessary for providing further validation and development of therapeutic applications.
CONFLICT OF INTEREST
The authors declare that they have no financial or non-financial conflict of interest regarding authorship and publication of this article..
ACKNOWLEDGMENTS
We are grateful to the Swami Vivekananda Merit Cum Means Scholarship [WBP201646991226] for partially funding this research.
AUTHORS’ CONTRIBUTION
AG participated in conceptualization, study design, methodology and wrote the original manuscript. TKK was involved in treatment protocol and histological analysis. SS was involved in extract preparation and biochemical analysis. AB was involved in ELISA and native gel expression study. SC was involved in supervision and manuscript editing. All authors reviewed the results and approved the final version of this present manuscript.

 10.2478/macvetrev-2025-0026
10.2478/macvetrev-2025-0026







