INTRODUCTION
Ehrlichiosis is a multisystemic disease that affects various organs and tissues, including the heart. Recent studies continue to focus on the cardiac effects of canine monocytic ehrlichiosis (CME). Gal et al. (2008) reported that
E. canis spreads to multiple organs, including the heart (
1). Varshney et al. (2015) suggested an increased risk of myocardial damage in acute CME cases (
2), while Charaborty et al. (
3) found that CME cases exhibited macroscopic and microscopic hemorrhages in the heart. Kumar and Varshney (
4) identified cardiac arrhythmias in canine ehrlichiosis but were unable to confirm the presence of myocardial damage.
Co-infections involving
Ehrlichia and
Babesia or
Trypanosoma have also been associated with significant arrhythmias (
4,
5). Electrocardiographic abnormalities such as sinus tachycardia, atrial fibrillation, second-degree Mobitz type B block, ventricular premature contractions (VPC), and ST changes are commonly found in dogs infected with CME (
6). Similar findings have been observed in human ehrlichiosis patients (
7).
The arrhythmias and conduction abnormalities in acute ehrlichiosis are thought to be linked to hypoxia and subsequent metabolic changes resulting from hemodynamic alterations (
4,
5). Severe arrhythmias, such as atrial fibrillation or multiple pathological electrocardiographic changes, may increase the risk of death in ehrlichiosis cases, emphasizing the need for prompt intervention. Despite the presence of arrhythmias in acute CME cases, electrocardiography (ECG) alone may not be sufficient to detect minor myocardial damage. Hence, cardiac biomarkers and echocardiography are crucial for early detection (
8). Biomarkers such as serum cardiac troponin I (cTnI), a sensitive indicator of myocardial injury, have been studied in ehrlichiosis-infected dogs. Elevated cTnI levels were observed in a significant number of cases, which showed a marked decrease after 14 days of treatment, suggesting that cardiac damage in
E. canis infection may be reversible (
8). Additionally, a study reported echocardiographic abnormalities in 33% of mono-infected, 27% of co-infected, and 34% of PCR-negative dogs, with left ventricular dilation and increased wall thickness correlated with elevated cTnI levels (
8). Anemia, which commonly affects multiple organs, can significantly influence cardiac function. Its impact is determined by factors such as anemia severity, onset rate, compensatory mechanisms, and the patient's physiological status (
9). Red blood cells play a key role in oxygen transport, and any imbalance can lead to reduced oxygen delivery, which the body attempts to compensate through mechanisms such as increased cardiac output and enhanced erythropoietin production (
10,
11).
The present study aimed to evaluate the severity of anemia, its related parameters, and its effects on cardiac function in dogs infected with
E. canis, a zoonotic pathogen of increasing importance. We hypothesized that the severity of anemia in
E. canis-infected dogs is associated with progressive echocardiographic and hematological alterations, reflecting compensatory cardiac remodeling and systemic involvement.
MATERIAL AND METHODS
Animal subjects and grouping
A total of 47 dogs were included in this study, consisting of 38 infected dogs and 9 healthy control dogs. The infected dogs, with no known history of cardiac disease, were presented to the Internal Medicine Clinic of the Faculty of Veterinary Medicine, Aydın Adnan Menderes University. These dogs exhibited clinical signs characteristic of different stages of canine monocytic ehrlichiosis (CME), such as fever, lethargy, anorexia, generalized lymphadenopathy, and epistaxis. The infected dogs were categorized based on anemia severity into five subgroups: very severe (n=2), severe (n=4), moderate (n=11), mild (n=9), and non-anemic (n=12). The severity of anemia was classified based on hematocrit (HCT) values as follows: very severe (<15%), severe (15–20%), moderate (21–25%), mild (26–30%), and non-anemic (>30%). The healthy control group consisted of 9 dogs, matched by age with the infected group. It is important to note that the number of dogs in the very severely anemic group was limited to n=2 due to case availability in the clinical population. Therefore, findings from this subgroup should be interpreted with caution and are intended to be descriptive rather than inferential.
The inclusion and exclusion criteria for animals were as follows:
1. Age between 1-8 years, avoiding immature or geriatric dogs;
2. Presence of clinical and pathological signs of CME, including lethargy, anorexia, fever, epistaxis, dyspnea, and splenomegaly;
3. No comorbidities such as hyperadrenocorticism, diabetes mellitus, hypothyroidism, or other protozoal diseases;
4. Not pregnant or lactating;
5. No history of chronic liver damage, congestive heart failure, or treatment with antihypertensive drugs;
6. Absence of pericardial effusion, congenital heart disease, or moderate to severe mitral or aortic valve insufficiency.
Healthy dogs were over one year of age, vaccinated, regularly treated for internal and external parasites, clinically healthy, and free from CME or other protozoal infections.
All dogs included in the infected group tested positive for
E. canis antibodies using the SNAP® 4Dx® ELISA test (IDEXX Laboratories, USA), which also displays for
Anaplasma spp.,
Borrelia burgdorferi, and
Dirofilaria immitis. Dogs that tested positive for any of these co-infections were excluded from the study. Additionally, SNAP Leishmania tests (IDEXX Laboratories, USA) were performed, and animals with positive results were excluded. In cases where clinical presentation was ambiguous, indirect fluorescent antibody test (IFAT) was applied to confirm
E. canis monoinfection with a cut-off titer: 1:50.
All procedures involving animals were conducted in accordance with national and institutional guidelines for the care and use of animals and were approved by the Local Ethics Committee for Animal Experiments (ADU-HADYEK), Aydın Adnan Menderes University, under approval number 64583101/2022/76. This study was supported by the Scientific Research Projects Coordination Unit of Aydın Adnan Menderes University under the project code VTF-23023.
Sample collection and laboratory analyses
Blood samples were collected from the cephalic vein (
V. cephalica antebrachii) of dogs presented to the Internal Medicine Clinic of Aydın Adnan Menderes University Veterinary Faculty. A total of 6 mL of blood was collected for analysis in separate tubes containing lithium heparin (4 mL) and EDTA (2 mL). Hematological analyses were performed using the Abacus Vet5 (Diatron®, Hungary), and anemia severity was determined. The remaining blood samples were centrifuged at 3,000 rpm for 15 min to obtain plasma samples, which were stored at -20 °C until cTnI levels were measured. Complete blood count was performed using the Abacus Vet5 (Hungary) device. Plasma samples were obtained by centrifugation of lithium heparin tubes at 3,000 rpm for 15 min. Biochemical analyses, including total iron, iron-binding capacity, and transferrin saturation, were conducted using enzymatic colorimetric methods with a Randox autoanalyzer (Randox Laboratories Ltd., UK). cTnI concentrations, a biomarker of myocardial injury, were measured using a fluorescent immunoassay device (Healvet Fia-3000, Healvet Medtech GZ Ltd.®, China). Test kits were stored according to the manufacturer's instructions, and testing procedures were performed within one hour of opening.
Echocardiographic evaluation
Following inclusion in the study, echocardiographic evaluations were conducted using a 5-7 MHz probe, performed by a single operator without the need for sedation to ensure consistency and minimize stress-related physiological variations. Imaging was acquired in both the long and short axes using B-mode and M-mode modalities, facilitating comprehensive assessment of ejection fraction, fractional shortening, and left ventricular dimensions during both systole and diastole. Additionally, mitral valve regurgitation was evaluated. Identical echocardiographic protocols were applied to the healthy control group to ensure comparability.
Statistical analysis
Descriptive statistics were performed for numerical variables, with normality assessed using standard diagnostic tests. The normality of data distribution was evaluated using Shapiro-Wilk tests. Depending on the distribution and homogeneity of variances, statistical comparisons between groups were performed using either one-way ANOVA followed by Tukey’s post-hoc test or the non-parametric Kruskal-Wallis test followed by Dunn’s test for multiple comparisons. Relationships between variables were evaluated using Pearson correlation coefficients. All statistical analyses were conducted using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA), and the significance level was set at p<0.05.
RESULTS
Clinical findings
The clinical evaluation of dogs presumptively diagnosed with CME revealed that cough was present in 9.1% of moderately anemic and 8.3% of severely anemic animals. Lethargy was absent in the control group but was observed in 25%, 77.8%, 81.8%, and 100% of non-anemic, mildly anemic, moderately anemic, and severely anemic dogs, respectively. Lymphadenopathy and bleeding manifestations, ranging from petechiae to epistaxis, were particularly notable in dogs with severe anemia. Dermatologic lesions, primarily characterized by exfoliative dermatitis and lunate formations, were prevalent in infected dogs, with a higher frequency among those with moderate to severe anemia, recorded at 63.6% and 75%, respectively. Poor body condition was noted exclusively in dogs with severe anemia.
Hematological findings
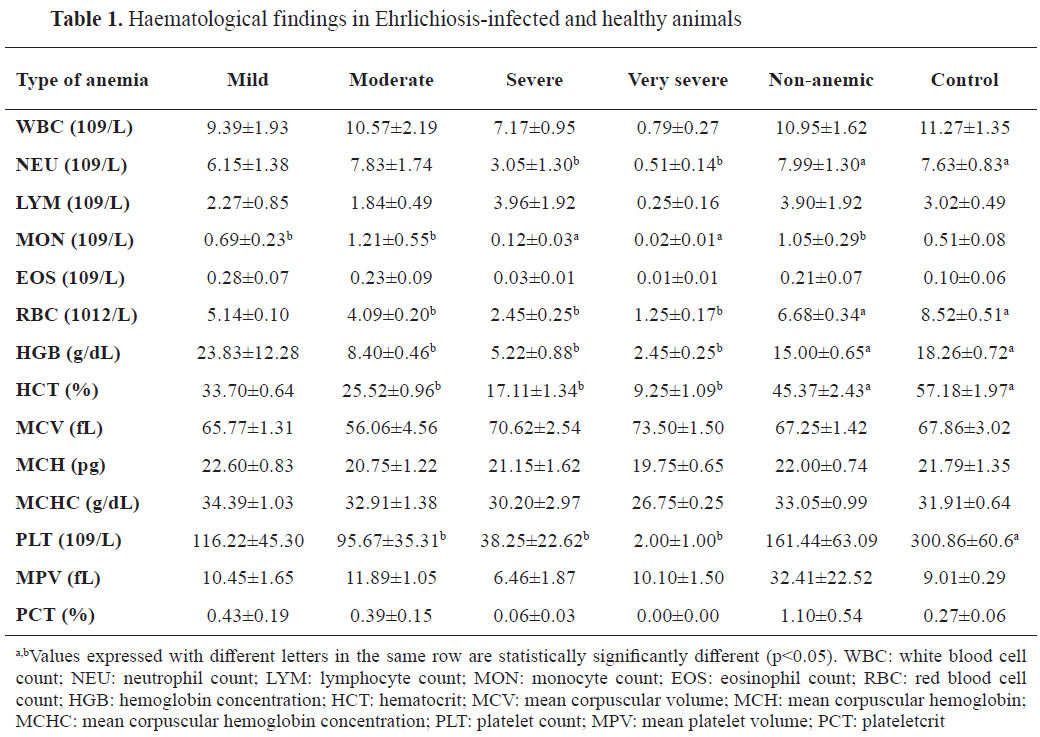
Hematological analyses revealed significant differences in anemia severity between the infected groups. Dogs in the very severe and severe anemia groups exhibited markedly lower hematocrit, hemoglobin, and red blood cell (RBC) counts compared to those in the mild and non-anemic groups (p<0.05). White blood cell (WBC) counts were elevated in the infected groups relative to the control group, suggesting an inflammatory response. Platelet counts were significantly reduced in infected dogs, particularly in those with severe anemia (p<0.01) (
Table 1).
Biochemical findings
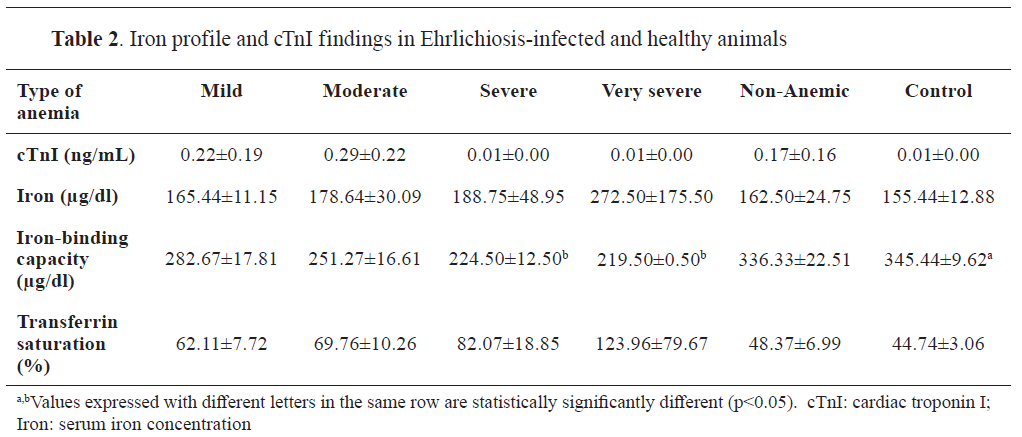
Biochemical analyses demonstrated that total iron levels and transferrin saturation were significantly lower in dogs with severe anemia compared to non-anemic and control groups (p<0.05). Iron-binding capacity was elevated in the severe anemia group, reflecting altered iron metabolism associated with the infection. Elevated cTnI levels were observed in infected dogs, with the highest mean concentration detected in the severely anemic group (0.29±0.22 ng/mL), significantly higher than in control animals (0.01±0.00 ng/mL) (p<0.01) (
Table 2).
Echocardiographic findings
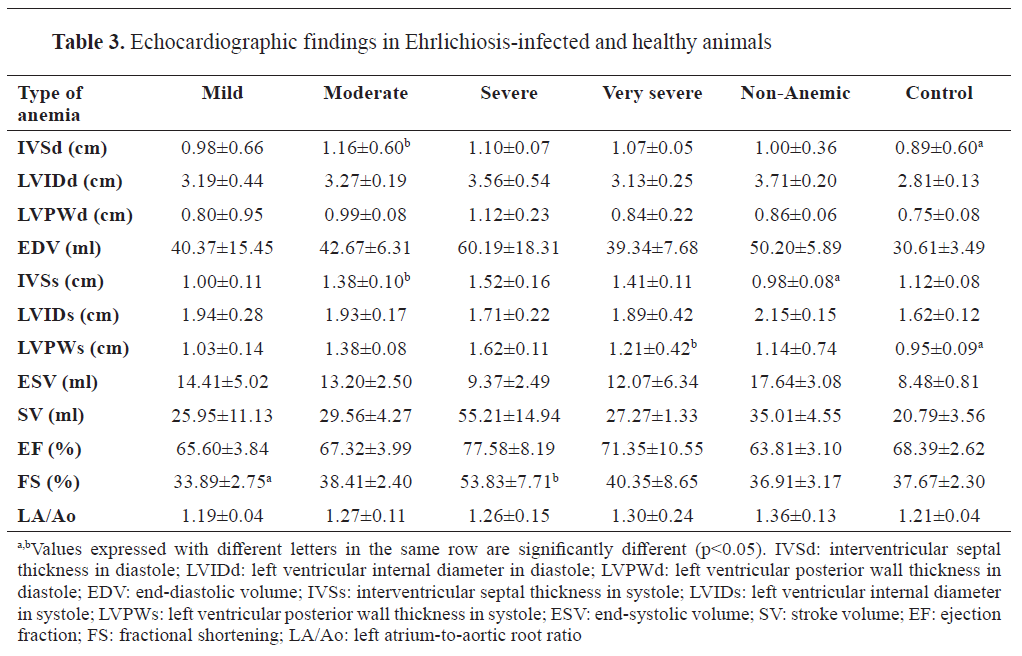
Echocardiographic evaluation revealed significant changes in cardiac dimensions and function among infected dogs. Dogs with severe anemia had increased left ventricular end-diastolic and end-systolic dimensions compared to the control group (p<0.05). Fractional shortening and ejection fraction were reduced in the severely anemic group, indicating compromised cardiac function. Mitral valve regurgitation was more prevalent in infected dogs, particularly those with moderate to severe anemia (
Table 3).
Correlation analysis
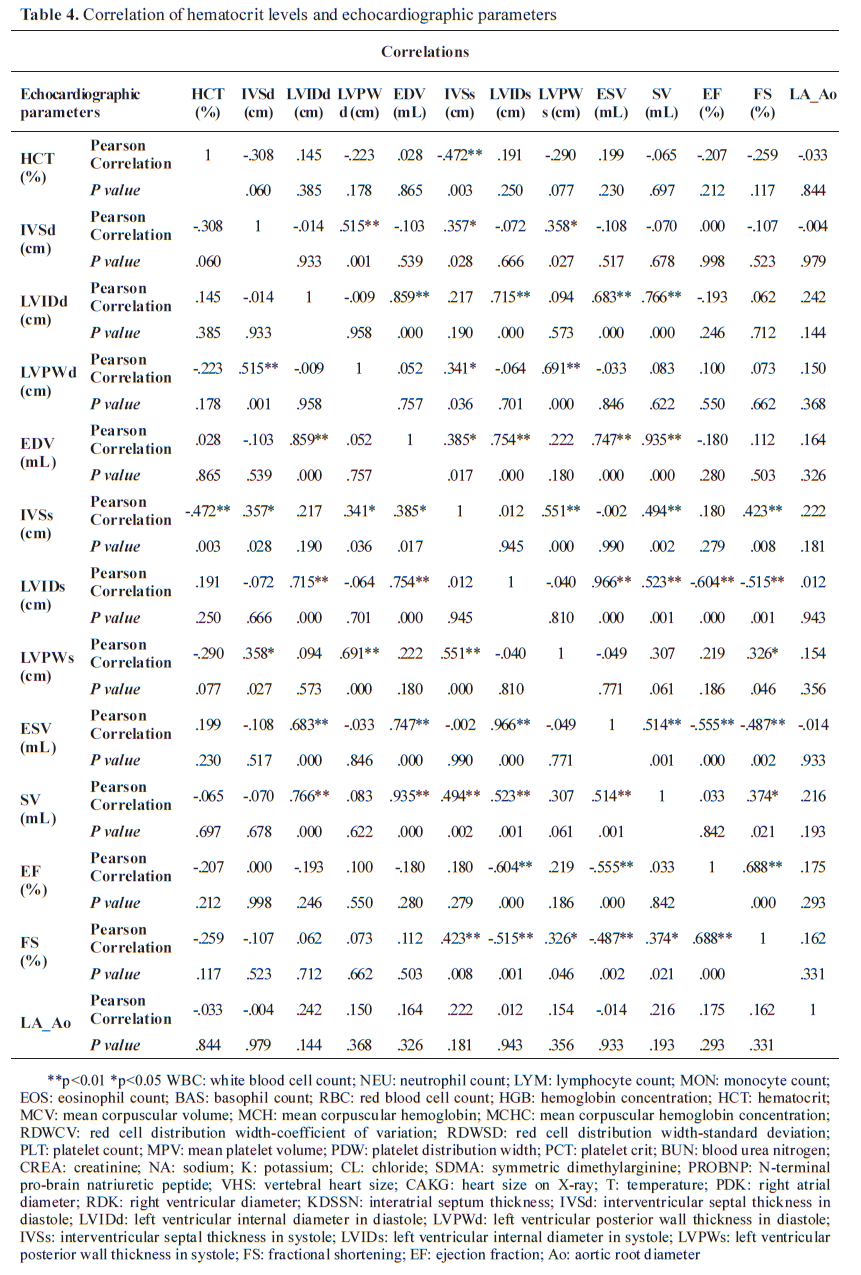
Pearson correlation analysis revealed a significant negative correlation between hematocrit levels and cTnI concentrations (r=-0.62, p<0.01). Additionally, left ventricular dimensions were positively correlated with cTnI levels (r=0.55, p<0.05) (
Table 4).
DISCUSSION
Canine Monocytic Ehrlichiosis (CME) is a globally distributed disease caused by the gramnegative, obligate intracellular bacterium
E. canis, primarily transmitted by the brown dog tick,
Rhipicephalus sanguineus (
12,
13,
14). Transmission could also be exhibited through blood transfusion from an infected donor (
15). The incubation period of
E. canis in dogs ranged from 8 to 20 days (
16). Typical clinical manifestations include high body temperature, ophthalmic lesions, epistaxis, and lymphadenopathy (
17). Other reported clinical signs include lethargy, depression, anorexia, hemorrhage, neurological symptoms, ocular inflammation, chronic weight loss, hind limb edema, pallor of mucous membranes, and cachexia (
17). The clinical findings in this study were consistent with previously reported data, with lethargy, lymphadenopathy, and exfoliative dermatitis being the most frequent signs, particularly in severely anemic cases. Anemia in CME can be attributed to several mechanisms, with mild to moderate anemia commonly observed during the acute phase as a result of inflammation-related processes. Chronic-phase anemia is typically characterized as normochromic, normocytic, and non-regenerative due to extensive bone marrow involvement (
18). Previous studies indicate that anemia severity escalates as the disease progresses to its chronic stage, evidenced by declining hematocrit levels (
19,
20). In this study, anemia presence and severity were determined based on positive
E. canis antibody detection via rapid test kits, which precluded differentiation of acute, chronic, or exposure phases, as well as antigen detection by PCR.
In the present study, hematocrit values were significantly lower in moderately, severely, and very severely anemic dogs compared to the non-anemic group, suggesting chronic phase involvement or prolonged exposure. Consistent with Kaewmongkol et al. (2017), the platelet counts in infected dogs included in the current study, exhibited a significant reduction in moderately to severely anemic groups compared to non-anemic or healthy controls (
19). Thrombocytopenia is a hallmark hematologic alteration in CME, particularly in the acute and chronic phases, characterized by significant platelet depletion, while subclinical cases often exhibit no platelet changes (
21). The development of pancytopenia during the chronic phase, associated with bone marrow suppression, further underscores the chronic nature of the disease in these cases. Our findings demonstrated a parallel between changes in hematocrit and platelet counts, with significantly lower platelet counts observed in moderately, severely, and very severely anemic dogs compared to non-anemic and healthy controls. These thrombocytopenic profiles align with previous reports emphasizing the role of thrombocytopenia in CME pathogenesis, underscoring its significance in diagnosing ehrlichiosis (
21,
22,
23).
Total leukocyte counts exhibited a decline from the non-anemic to the very severely anemic groups, although no significant differences were noted between healthy and infected groups. Neutrophil counts were significantly reduced in the severely and very severely anemic groups compared to the non-anemic and healthy controls, which is in agreement with the findings by Dixit et al. (
24). However, De Castro et al. (
25) reported contrasting results, with significantly elevated neutrophil levels in infected dogs compared to the healthy control, possibly attributed to underlying co-infections.
Iron metabolism is pivotal in the pathophysiology of CME, with approximately 60-70% of total body iron contained in hemoglobin (
26,
27). Disruptions in iron absorption, utilization, or loss can result in conditions such as iron deficiency anemia or anemia due to inflammation (28). In this study, serum iron, iron-binding capacity, and transferrin saturation were assessed across different anemia severity groups. No significant differences were found among the groups, likely due to the chronic or indolent nature of the disease, which allows iron stores to be maintained at adequate levels.
Myocardial injury is a recognized sequela of CME, frequently indicated by elevated cardiac troponin I (cTnI) levels, implying multisystemic involvement (
29). Previous studies have reported myocardial damage in naturally infected dogs, reflected by increased cTnI levels (
2,
30). In our study, elevated cTnI levels were observed in only a limited number of cases, suggesting that myocardial injury was not pervasive among the examined animals. The stage of infection-acute or chronic-may influence cTnI elevation, as acute myocardial injury can be detected within four hours of ischemic events (
31). This could briefly explain our results obtained at this study.
Echocardiographic evaluation in the current study revealed that interventricular septal thickness during diastole (IVSd) was significantly greater in moderately anemic dogs compared to the healthy group, suggesting a compensatory adaptation to anemia. This increased thickness was also observed during systole (IVSs) in moderately anemic dogs compared to non-anemic infected and healthy dogs. Additionally, the fractional shortening (FS) percentage was higher in the severely anemic group compared to the mildly anemic group. These findings imply that myocardial changes are associated with anemia severity and may represent ongoing compensatory mechanisms in response to chronic anemia (
32,
33). Consistent with previous studies, the echocardiographic parameters indicated hyperdynamic cardiac function, characterized by increased systolic performance while maintaining diastolic function (
32,
33).
To conclude, our findings demonstrate that anemia severity was closely correlated with both myocardial and hematological alterations in
E. canis-infected dogs. The observed echocardiographic changes further support the presence of compensatory mechanisms in response to chronic anemia. Although the number of animals in the very severely anemic group was limited, their inclusion still provided valuable observational insight into extreme clinical cases. Future studies employing controlled infection models and larger sample sizes are essential to better understand the progression of myocardial injury and the influence of co-infections in ehrlichiosis.
CONCLUSION
Differences were found on some parameters from cardiac and hematological outcomes depending on the severity of anemia. No significant cTnI differences were found, but echocardiography showed higher IVSd and IVSs in moderately anemic dogs, suggesting early compensatory remodeling. Fractional shortening was greater in severely anemic dogs, indicating enhanced cardiac function as a compensatory response. Chronic anemia may induce cardiac changes that could progress to dysfunction, emphasizing early intervention.
E. canis-infected dogs may present with cardiac abnormalities like left ventricular hypertrophy. Comprehensive studies using advanced diagnostics, such as cardiac MRI and Doppler echocardiography, are needed to understand disease progression.
CONFLICT OF INTEREST
The authors declare that they have no financial or non-financial conflict of interest regarding authorship and publication of this article.
ACKNOWLEDGMENTS
This study is a summary of a section from the Master's thesis entitled "Investigation of Echocardiographic and Hematological Changes in Naturally Infected Dogs With Ehrlichiosis" conducted at the Department of Internal Medicine, Institute of Health Sciences, Aydın Adnan Menderes University. It was supported by Aydın Adnan Menderes University Scientific Research Projects Unit under project number VTF-23023.
AUTHORS’ CONTRIBUTION
All authors made the conceptualization and methodology of the study. HE and ZU were responsible for the project administration. ZU, TÖ, SE and HE conducted the investigation and data curation. ZE, KU and HE performed the formal analysis. All authors were included in writing of the original draft, review and editing of the manuscript.

 10.2478/macvetrev-2025-0027
10.2478/macvetrev-2025-0027