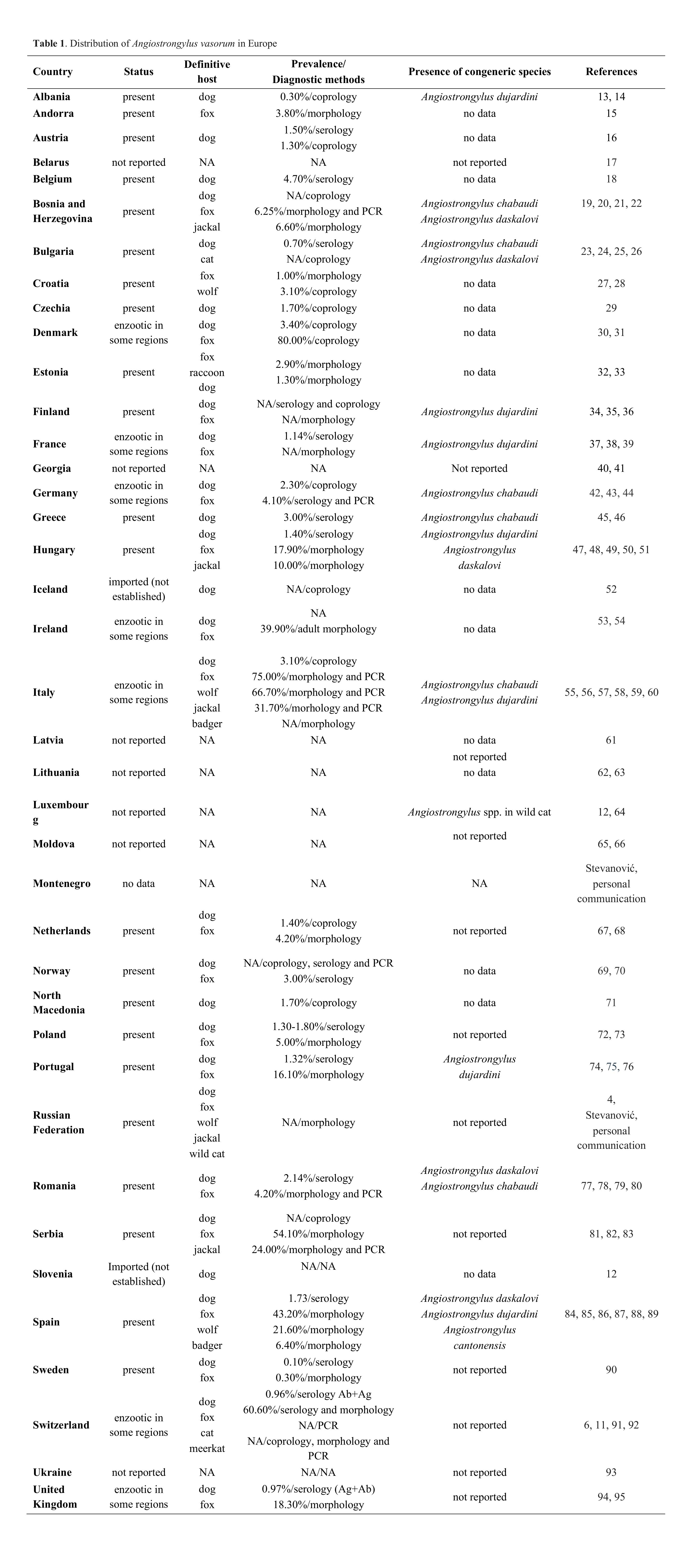
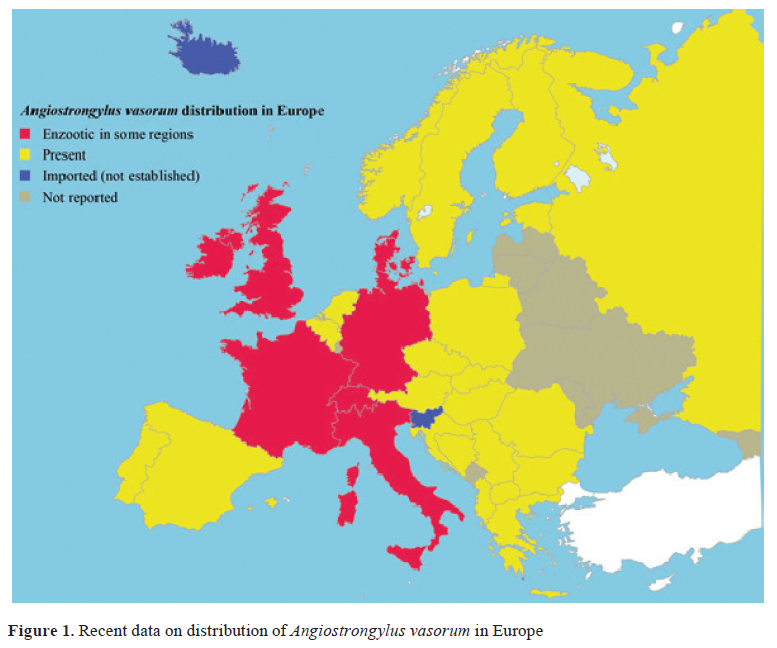
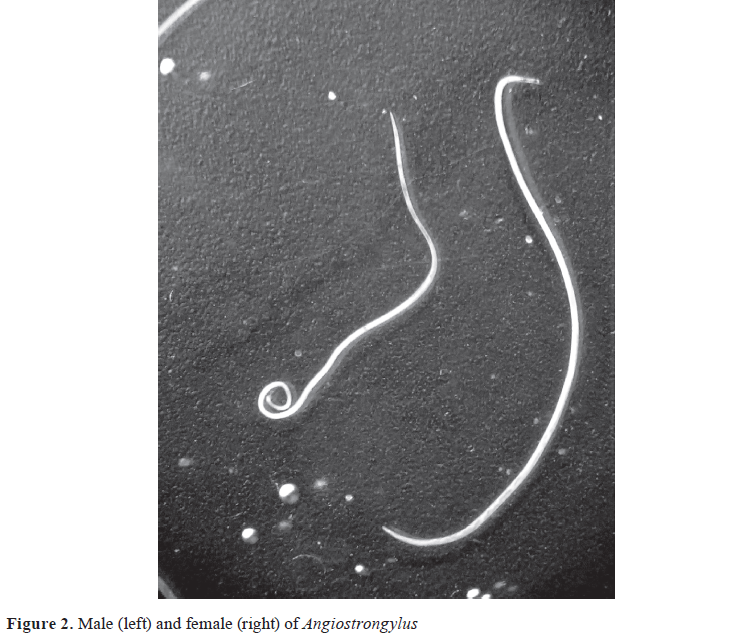
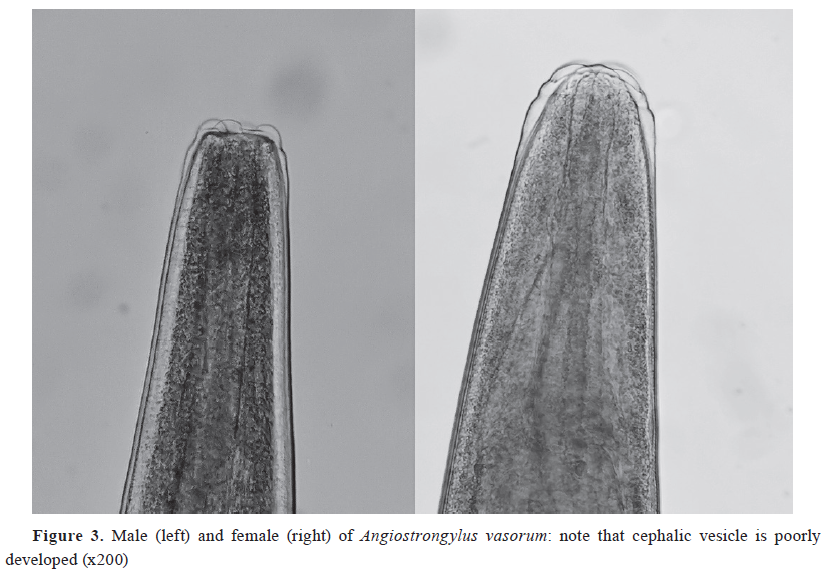
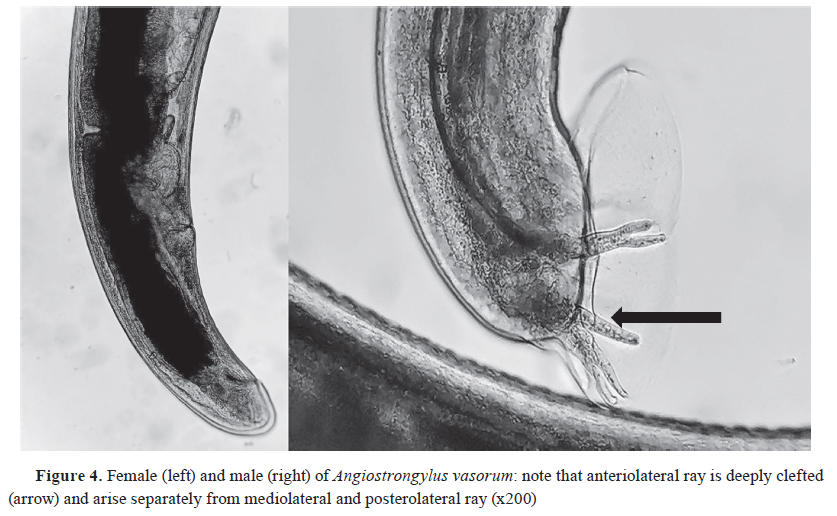
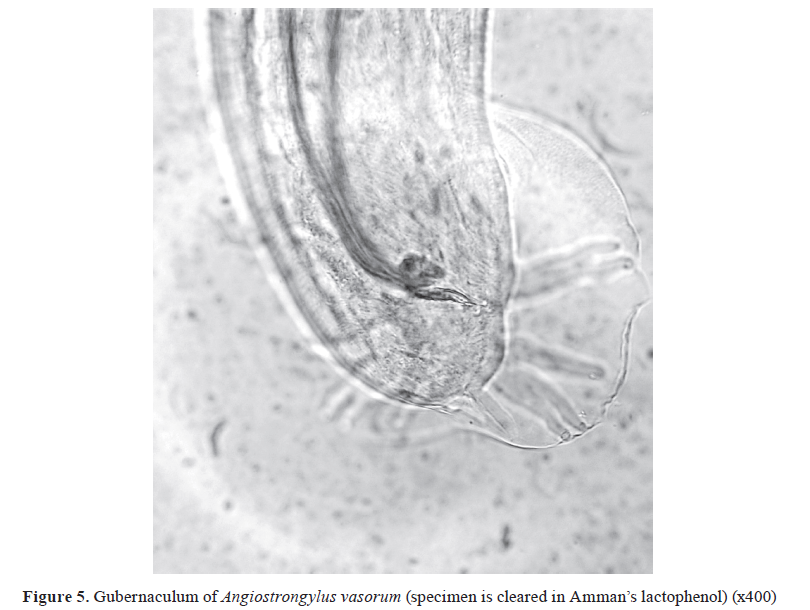
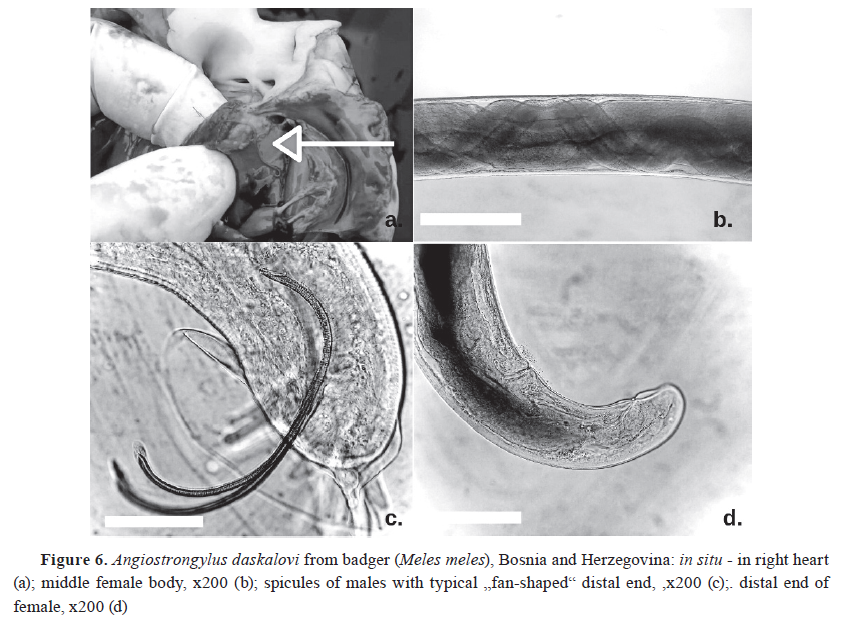
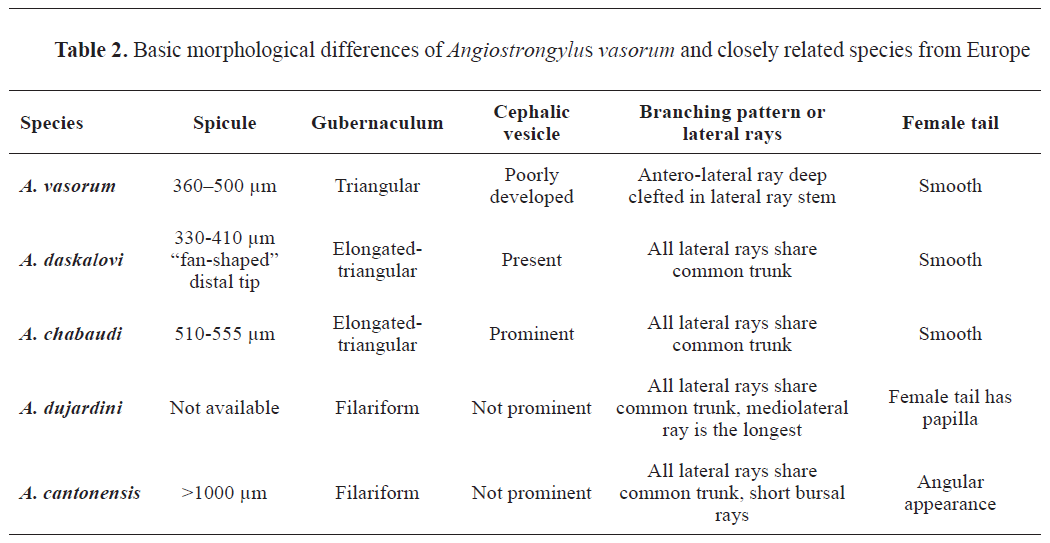
-
Baillet, C. (1957). Strongyle of the vessels and the heart of the dog Strongylus vasorum. [Strongle des vaisseaus et du coeur du chein Strongylus vasorum (Nobis).] N Dict Paract Med Vet. 8, 587-588. [In French]
-
Morgan, ER, Modrý, D., Paredes-Esquivel, C., Foronda, P., Traversa, D. (2021). Angiostrongylosis in animals and humans in Europe. Pathogens. 10(10): 1236. https://doi.org/10.3390/pathogens10101236 PMid:34684185 PMCid:PMC8538298
-
Spratt, M. (2015). Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: A review. Int J Parasitol Parasites Wildl. 4(2): 178-189. https://doi.org/10.1016/j.ijppaw.2015.02.006 PMid:25853051 PMCid:PMC4381133
-
Kozlov, P. (1977). Key to the helminths of carnivores in the USSR. Science. 276.
-
Cowie, H. (2019). Annotated catalog of species of Angiostrongylus and the related genera Gallegostrongylus, Rodentocaulus and Stefanskostrongylus (Nematoda: Metastrongyloidea, Angiostrongylidae). J Helminthol. 93(4): 389-423. https://doi.org/10.1017/S0022149X19000270 PMid:31064435
-
Gueldner, K., Schuppisser, C., Borel, N., Hilbe, M., Schnyder, M. (2019). First case of a natural infection in a domestic cat (Felis catus) with the canid heart worm Angiostrongylus vasorum. Vet Parasitol Reg Stud Reports. 18, 100342. https://doi.org/10.1016/j.vprsr.2019.100342 PMid:31796174 PMCid:PMC7104072
-
Riese, K., Baker, E., Dennis, MM, Williamson, R., Gerhold, R. (2024). Two cases of Angiostrongylus vasorum, a cardiopulmonary nematode, in a wild black bear and coyote of Vet Parasitol Reg Stud Reports. 54, 101079. https://doi.org/10.1016/j.vprsr.2024.101079 PMid:39237243
-
Mechouck, Deak, G., Ionică, AM, Toma, C., Negoescu, AG, Taulescu, M., Bouslama, Z., Mihalca, AD (2024). First report of Angiostrongylus vasorum in an African golden wolf (Canis lupaster) in Algeria. Parasite Vectors. 17(1): 441. https://doi.org/10.1186/s13071-024-06534-9 PMid:39468624 PMCid:PMC11520683
-
Robleto-Quesada, J., Umaña-Blanco, F., Solano-Barquero, A., Allen, J., Levi, T., Gori, F., Schnyder, M., Rojas, A. (2024). Seek, and you will find: Cryptic diversity of the cardiopulmonary nematode Angiostrongylus vasorum in the Americas. Acta Trop. 258, 107337.
https://doi.org/10.1016/j.actatropica.2024.107337 PMid:39098751
-
Karadjian, G., Kaestner, C., Laboutière, L., Adicéam, E., Wagner, T., Johne, A., Thomas, M., et al. (2020). A two-step morphology-PCR strategy for the identification of nematode larvae recovered from muscles after artificial digestion at meat inspection. Parasitol Res. 119(12): 4113-4122.
https://doi.org/10.1007/s00436-020-06899-7 PMid:32979104
-
Gillis-Germitsch, N., Tritten, L., Hegglin, D., Deplazes, P., Schnyder, M. (2020). Conquering Switzerland: the emergence of Angiostrongylus vasorum in foxes over three decades and its rapid regional increase in prevalence contrast with the stable occurrence of lungworms. Parasitol. 147(10): 1071-1079.
https://doi.org/10.1017/S0031182020000700 PMid:32372743 PMCid:PMC10317728
-
Fuehrer, P., Morelli, S., Unterköfler, M.S., Bajer, A., Bakran-Lebl, K., Dwużnik-Szarek, D., Farkas, R., et al. (2021). Dirofilaria spp. and Angiostrongylus vasorum: Current Risk of Spreading in Central and Northern Europe. Pathogens. 10(10): 1268.
https://doi.org/10.3390/pathogens10101268 PMid:34684217 PMCid:PMC8537668
-
Shukullari, E., Hamel, D., Rapti, D., Pfister, K., Visser, M., Winter, R., Rehbein, S. (2015). Parasites and vector-borne diseases in client-owned dogs in Albania. Intestinal and pulmonary endoparasite infections. Parasitol Res. 114(12): 4579-4590.
https://doi.org/10.1007/s00436-015-4704-8 PMid:26350379
-
Kontrimavichus, V.L. (1979). Subfamily Angiostrongylinae Boehm and Gebaur, In: V.L. Kontrimavichus, S.L. Delyamure, (Eds.), Principles of nematology. Volume 29. Filaroids of domestic and wild animals (pp. 55-138). Moscow: Nauka Publishers
-
Segovia, M., Torres, J., Miquel, J. (2004). Helminth parasites of the red fox (Vulpes vulpes L., 1758) in the Iberian Peninsula: an ecological study. Acta Parasitol. 49(1): 67-79.
-
Globokar, M., Pantchev, N., Hinney, B., Leschnik, M., Peschke, R., Schaper, R., Schnyder, M. (2021). Serological and faecal detection of Angiostrongylus vasorum in dogs from Austria. Vet Parasitol Reg Stud Reports. 26, 100641.
https://doi.org/10.1016/j.vprsr.2021.100641 PMid:34879952
-
-
Lempereur, L., Martinelle, L., Marechal, F., Bayrou, C., Dalemans, A.C., Schnyder, M., Losson, B. (2016). Prevalence of Angiostrongylus vasorum in southern Belgium, a coprological and serological Parasit Vectors. 9(1): 533.
https://doi.org/10.1186/s13071-016-1820-y PMid:27716374 PMCid:PMC5052934
-
Stevanović, O., Dobrijević, M., Vujanić, D., Nedić, D., Ilić, , Trbojević, I. (2020). First report of autochthonous canine angiostrongylosis in Bosnia and Herzegovina. Vet Glasnik. 7(1): 85-91.
https://doi.org/10.2298/VETGL2001085S
-
Stevanović, O., Diakou, A., Morelli, S., Paraš, S., Trbojević, , Nedić, D., Sladojević, Ž., et al. (2019). Severe verminous pneumonia caused by natural mixed infection with Aelurostrongylus abstrusus and Angiostrongylus chabaudi in a European wild cat from Western Balkan area. Acta Parasitol. 64(2): 411-417.
https://doi.org/10.2478/s11686-019-00029-9 PMid:30756237
-
Stevanović, O., Vujanić, D., Dobrijević, M., Trbojević, I., Gajić, N., Nedić, D., Sladojević, Ž. (2019). Cardiorespiratory nematode in carnivores from Republic of Srpska, Lecture by Invitation. 24rd Annual Counselling of Doctors of Veterinary Medicine of Republic of Srpska (Bosnia and Herzegovina), 81-82. [In Serbian]
-
Stevanović, , Despotović, D., Ilić, T., Jovanović, N., Trbojević, I., Radalj, A. (2024). Angiostrongylus vasorum in golden jackals (Canis aureus) and red foxes (Vulpes vulpes) from Northern Bosnia and Herzegovina. Parasitol Res. 123, 352.
https://doi.org/10.1007/s00436-024-08377-w PMid:39414642
-
Kamenov, Y., Radev, V., Zlateva, N. (1999). On diagnosis and clinic of angiostrongylosis of cats. Exp Pathol Parasitol. 2, 51-54.
-
Pantchev, N., Schnyder, M., Vrhovec, M.G., Schaper, R., Tsachev, I. (2015). Current surveys of the seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in dogs in Bulgaria. Parasitol Res. 114(Suppl. 1): 117-130.
https://doi.org/10.1007/s00436-015-4518-8 PMid:26152413
-
Panayotova-Pencheva, M., Trifonova, A., Dakova, V., Salkova, D., Movsesyan, S.O. (2017). On the parasites of genus Angiostrongylus (Nematoda: Angiostrongylidae) and some cases of Angiostrongylus daskalovi in badgers from Bulgaria. Russ J Parasitol. 40(2): 124-128.
-
Giannelli, A., Kirkova, Z., Abramo, F., Latrofa, M.S., Campbell, B., Zizzo, N., Cantacessi, C., et al. (2016). Angiostrongylus chabaudi in felids: New findings and a review of the literature. Vet Parasitol. 228, 188-192.
https://doi.org/10.1016/j.vetpar.2016.09.007 PMid:27692325
-
Rajković-Janje, R., Marinculić, A., Bosnić, S., Benić, M., Vinković, B., Mihajlević, Ž. (2003). Prevalence and seasonal distribution of helminth parasites in red foxes (Vulpes vulpes) from the Zagreb County (Croatia). Z Jagdwiss. 48, 151-160.
https://doi.org/10.1007/BF02189989
-
Hermosilla, C., Kleinertz, S., Silva, L.M., Hirzmann, J., Huber, D., Kusak, J.,Taubert, A. (2017). Protozoan and helminth parasite fauna of free-living Croatian wild wolves (Canis lupus) analyzed by scat collection. Vet Parasitol. 233, 14-19.
https://doi.org/10.1016/j.vetpar.2016.11.011 PMid:28043382
-
Vernerová, E., Dvorakova, N., Svobodová, V., Bures, J. (2020). Factors affecting the occurrence of gastrointestinal parasites and lungworms in dogs and assessment of antiparasitic drugs use patterns. Acta Vet Brno. 91(2): 171-177.
https://doi.org/10.2754/avb202291020171
-
Lundsgaard, K. (2024). Epidemiology of Angiostrongylus vasorum and Crenosoma vulpis infection in dogs in Denmark. J Small Anim Pract. 65(10): 737-748.
https://doi.org/10.1111/jsap.13762 PMid:38973253
-
Bolt, G., Monrad, J., Henriksen, P., Dietz, H.H., Koch, J., Bindseil, E., Jensen, A.L. (1992). The fox (Vulpes vulpes) as a reservoir for canine angiostrongylosis in Denmark. Field survey and experimental infections. Acta Vet Scand. 33(4): 357-362.
https://doi.org/10.1186/BF03547302 PMid:1488951 PMCid:PMC8117834
-
Laurimaa, L., Moks, E., Soe, E., Valdmann, H., Saarma, U. (2016). Echinococcus multilocularis and other zoonotic parasites in red foxes in Estonia. Parasitol. 143(11): 1450-1458.
https://doi.org/10.1017/S0031182016001013 PMid:27279259
-
Laurimaa, L., Süld, K., Davison, J., Moks, E., Valdmann, , Saarma, U. (2016). Alien species and their zoonotic parasites in native and introduced ranges: The raccoon dog example. Vet Parasitol. 219, 24-33.
https://doi.org/10.1016/j.vetpar.2016.01.020 PMid:26921035
-
Tiškina, V., Lindqvist, E.L., Blomqvist, A.C., Orav, M., Stensvold, C.R., Jokelainen, P. (2019). Autochthonous Angiostrongylus vasorum in Finland. Vet Rec Open. 6(1): e000314.
https://doi.org/10.1136/vetreco-2018-000314 PMid:30997112 PMCid:PMC6446214
-
-
Tenora, F., Henttonen, H., Haukisalmi, V. (1983). On helminths of rodents in Finland. Ann Zool Fenn. 20, 37-45.
-
Schnyder, M., Bilbrough, G., Hafner, C., Schaper, R. (2017). Angiostrongylus vasorum, ‘The French heartworm’: a serological survey in dogs from France introduced by a brief historical review. Parasitol Res. 116(Suppl. 1): 31-40.
https://doi.org/10.1007/s00436-017-5489-8 PMid:28717947
-
-
Graille, M., Ferté, H., Petit, T., Courtois, F.O., Gauchot, J.Y., Nougaillon, J.L., Vitaud, C., et al. (2015). Fatal Parastrongylus dujardini infection in captive callitrichids. Vet Pathol. 52(2): 364-368.
https://doi.org/10.1177/0300985814531496 PMid:24793826
-
Pochverya, O., Shikvishvily, M.I., Gvaladze, A.Z., Turmanidze, M.O. (2015). On the issue of helminth infection in dogs of the population of Tbilisi. Theory and Practice of Combating Parasitic Diseases [К вопросу об инвазированности гельминтами собак населения г. Тбилиси.Теория и практика борьбы с паразитарными болезнями.] 16, 352-353. [In Russian]
-
Кurashvili, Е., Matsaberidze, G.V., Sadikhov, I.А., Rodonaia, T.E. (1989). Parasitic worms of small mammals of the Transcaucasian fauna. Metsniereba, Tbilisi, 196.
-
Maksimov, P., Hermosilla, , Taubert, A., Staubach, C., Sauter-Louis, C., Conraths, F.J., Vrhovec, M.G., Pantchev, N. (2017). GIS-supported epidemiological analysis on canine Angiostrongylus vasorum and Crenosoma vulpis infections in Germany. Parasit Vectors. 10(1): 108.
https://doi.org/10.1186/s13071-017-2054-3 PMid:28241853 PMCid:PMC5330135
-
Schug, K., Krämer, F., Schaper, R., Hirzmann, J., Failing, K., Hermosilla, C., Taubert, A. (2018). Prevalence survey on lungworm (Angiostrongylus vasorum, Crenosoma vulpis, Eucoleus aerophilus) infections of wild red foxes (Vulpes vulpes) in central Germany. Parasit Vectors. 11(1): 85.
https://doi.org/10.1186/s13071-018-2672-4 PMid:29409523 PMCid:PMC5801722
-
Bisterfeld, K., Raulf, M.K., Waindok, P., Springer, A., Lang, J., Lierz, M., Siebert, U., Strube, C. (2022). Cardio-pulmonary parasites of the European wildcat (Felis silvestris) in Germany. Parasit Vectors. 15(1): 452.
https://doi.org/10.1186/s13071-022-05578-z PMid:36471378 PMCid:PMC9724372
-
Angelou, A., Gelasakis, A.I, Schnyder, M., Schaper, R., Papadopoulos, E. (2020). The ‘French heartworm’ in Greece: A countrywide serological survey of Angiostrongylus vasorum infection by combined detection of circulating antigens and specific antibodies. Vet Parasitol Reg Stud Reports. 19, 100376.
https://doi.org/10.1016/j.vprsr.2020.100376 PMid:32057383
-
Diakou, A., Psalla, D., Migli, D., Di Cesare, A., Youlatos, D., Marcer, F., Traversa, D. (2016). First evidence of the European wildcat (Felis silvestris silvestris) as definitive host of Angiostrongylus chabaudi. Parasitol Res. 115(3): 1235-1244.
https://doi.org/10.1007/s00436-015-4860-x PMid:26637312
-
Schnyder, M., Schaper, R., Lukács, Z., Hornok, S., Farkas, (2015). Combined serological detection of circulating Angiostrongylus vasorum antigen and parasite-specific antibodies in dogs from Hungary. Parasitol Res. 114(Suppl. 1): 145-154.
https://doi.org/10.1007/s00436-015-4520-1 PMid:26152415
-
Tolnai, Z., Széll, Z., Sréter, T. (2015). Environmental determinants of the spatial distribution of Angiostrongylus vasorum, Crenosoma vulpis and Eucoleus aerophilus in Hungary. Vet Parasitol. 207(3-4): 355-358.
https://doi.org/10.1016/j.vetpar.2014.12.008 PMid:25547643
-
Takács, A., Szabó, L., Juhász, L., Takács, A., Lanszki, J., Takács, P., Heltai, M. (2014). Data on the parasitological status of golden jackal (Canis aureus L., 1758) in Hungary. Acta Vet Hung. 62(1): 33-41.
https://doi.org/10.1556/avet.2013.058 PMid:24334089
-
Mészáros, F. (1972). The occurrence of Angiostrongylus (P.) dujardini Drozdz et Doby, 1970 (Nematoda) in rodents in Hungary. Parasitol Hung. 5, 163-176.
-
Nagy, E., Benedek, I., Zsolnai, A., Halász, T., Csivincsik, Á., Ács, V., Nagy, G., Tari, T. (2021). Habitat characteristics as potential drivers of the Angiostrongylus daskalovi infection in European badger (Meles meles) populations. Pathogens. 10(6): 715.
https://doi.org/10.3390/pathogens10060715 PMid:34200340 PMCid:PMC8228055
-
Skirnisson, K., Pálsdóttir, G.R., Eydal, M. (2018). Parasites of dogs and cats imported to Iceland during 1989-2017 with remarks on parasites occurring in the native populations. Icel Agric Sci. 31, 49-63.
https://doi.org/10.16886/IAS.2018.04
-
Gallagher, B., Brennan, S.F., Zarelli, M., Mooney, C.T. (2012). Geographical, clinical, clinicopathological and radiographic features of canine angiostrongylosis in Irish dogs: a retrospective study. Ir Vet J. 65, 5.
https://doi.org/10.1186/2046-0481-65-5 PMid:22433388 PMCid:PMC3349590
-
McCarthy, G., Ferrand, M., De Waal, T., Zintl, A., McCrath, , Byrne, W., O’Neill, E.J. (2016). Geographical distribution of Angiostrongylus vasorum in foxes (Vulpes vulpes) in the Republic of Ireland. Parasitol. 143(5): 588-593.
https://doi.org/10.1017/S0031182016000032 PMid:26940534
-
Traversa, D., Morelli, S., Di Cesare, A., Astuti, C., Barlaam, , Colombo, M., Veronesi, F., et al. (2025). Current Enzooticity of Dirofilaria immitis and Angiostrongylus vasorum in Central and Southern Italy. Animals (Basel). 15(2): 172.
https://doi.org/10.3390/ani15020172 PMid:39858171 PMCid:PMC11758344
-
Tieri, E.E., Saletti, A., D’Angelo, A.R., Parisciani, G., Pelini, S., Cocco, A., Di Teodoro, G., et al. (2021). Angiostrongylus vasorum in foxes (Vulpes vulpes) and wolves (Canis lupus italicus) from Abruzzo region, Italy. Int J Parasitol Parasites Wildl. 15, 184-194.
https://doi.org/10.1016/j.ijppaw.2021.05.003 PMid:34136344 PMCid:PMC8182381
-
Orioles, M., Fabbri, D., Miani, G., Pesaro, S., Dorigo, L., Bregoli, M., Saccà, E., et al. (2024). Double trouble: Co-infection of Angiostrongylus vasorum and Dirofilaria immitis in golden jackal (Canis aureus) in Friuli Venezia Giulia, Italy. Int J Parasitol Parasites Wildl. 24, 100969.
https://doi.org/10.1016/j.ijppaw.2024.100969 PMid:39165606 PMCid:PMC11334653
-
Varcasia, A., Tamponi, C., Brianti, E., Cabras, P.A., Boi, R., Pipia, A.P., Giannelli, A., et al. (2014). Angiostrongylus chabaudi Biocca, 1957: a new parasite for domestic cats? Parasit Vectors. 7, 588.
https://doi.org/10.1186/s13071-014-0588-1 PMid:25515026 PMCid:PMC4308007
-
Eleni, C., Di Cesare, A., Cavicchio, P., Tonnicchia, M.C., Meoli, R., di Regalbono, A.F., Paoletti, B., et al. (2016). Fatal Angiostrongylus dujardini infection in callitrichid monkeys and suricates in an Italian zoological garden. Parasitol Int. 65(4): 333-335.
https://doi.org/10.1016/j.parint.2016.04.005 PMid:27094227
-
Magi, M., Guardone, L., Dell’Omodarme, M., Prati, M., Mignone, W., Torracca, B., Monni, G., Macchioni, F. (2009). Angiostrongylus vasorum in red foxes (Vulpes vulpes) and badgers (Meles meles) from Central and Northern Italy. Hystrix It J Mamm. 20(2): 121-126.
-
-
Bružinskaitė-Schmidhalter, R., Šarkūnas, M., Malakauskas, A., Mathis, A., Torgerson, P.R., Deplazes, P. (2012). Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitol. 139(1): 120-127.
https://doi.org/10.1017/S0031182011001715 PMid:21996514
-
Nugaraitė, D., Mažeika, V., Paulauskas, A. (2019). Helminths of Mustelids with Overlapping Ecological Niches: Eurasian Otter Lutra Lutra (Linnaeus, 1758), American Mink Neovison Vison Schreber, 1777, and European Polecat Mustela Putorius Linnaeus, 1758. Helminthologia. 56(1): 66-74.
https://doi.org/10.2478/helm-2018-0035 PMid:31662675 PMCid:PMC6662030
-
Steeb, (2015). Postmortem examinations of the European wildcat (Felis silvestris silvestris Schreber, 1777). [Postmortale Untersuchungen an der Europäischen Wildkatze (Felis silvestris silvestris SCHREBER, 1777).] PhD thesis, Justus Liebig Universität, Fachbereich Veterinärmedizin, Gießen, VVB Laufersweiler Verlag Gießen, p. 230. [In German]
-
Chihai, O., Nistreanu, V., Larion, A., Tălămbuţă, N., Rusu, Ş., Zamornea, M., Melnic, G. (2023). Transmissible parasitic zoonoses of the species Vulpes vulpes (Linnaeus, 1758). Natural sciences. In: The dialogue of generations, September, 14-15 (p. 86), Chisinau, Republic of Moldova
-
Chihai, O., Nistreanu, V., Larion, A., Rusu, Ş., Tălămbuţă, N., Zamornea, M., Melnic, G., Kolodrevski, O. (2022). Epidemiological characteristics of the parasite fauna in Apodemus uralensis (Pallas, 1771) from various biotopes of the Republic of [Caracteristica epidemiologică a parazitofaunei la Apodemus uralensis (Pallas, 1771) din diverse biotopuri ale Republicii Moldova.] Studia Universitatis Moldaviae (Seria Ştiinţe Reale şi ale Naturii), 156(6): 81-86. [In Romanian]
-
Lempereur, L., Nijsse, R., Losson, B., Marechal, F., De Volder, , Schoormans, A., Martinelle, L., et al. (2020). Coprological survey of endoparasite infections in owned dogs and owners’ perceptions of endoparasite control in Belgium and the Netherlands. Vet Parasitol Reg Stud Reports. 22, 100450.
https://doi.org/10.1016/j.vprsr.2020.100450 PMid:33308762
-
Franssen, F., Nijsse, R., Mulder, J., Cremers, H., Dam, C., Takumi, K., van der Giessen, J. (2014). Increase in number of helminth species from Dutch red foxes over a 35-year period. Parasit Vectors. 7, 166.
https://doi.org/10.1186/1756-3305-7-166 PMid:24708710 PMCid:PMC3978201
-
Robbestad, J., Jiménez-Meléndez, A., Robertson, L.J., Vatne, L.I., Hauback, M.N., Nerhagen, S. (2024). First case of autochthonous Angiostrongylus vasorum infection in a Norwegian dog. Acta Vet Scand. 66(1): 41.
https://doi.org/10.1186/s13028-024-00765-7 PMid:39223595 PMCid:PMC11367999
-
Hamnes, I.S., Enemark, H.L., Henriksen, K., Madslien, K., Er, C. (2019). The surveillance programme for Angiostrongylus vasorum in red foxes (Vulpes vulpes) Norway 2019. Norwegian Veterinary Institute 2020 - Annual Report. 1-7.
-
-
Schnyder, M., Schaper, R., Pantchev, N., Kowalska, D., Szwedko, A., Deplazes, P. (2013). Serological detection of circulating Angiostrongylus vasorum antigen-and parasite-specific antibodies in dogs from Poland. Parasitol Res. 112(Suppl. 1): 109-117.
https://doi.org/10.1007/s00436-013-3285-7 PMid:23779223
-
Demiaszkiewicz, W., Pyziel, A.M., Kuligowska, I., Lachowicz, J., Demiaszkiewicz, A.W., Pyziel, A.M. (2014). The first report of Angiostrongylus vasorum (Nematoda; Metastrongyloidea) in Poland, in red foxes (Vulpes vulpes). Acta Parasitol. 59(4): 758-762.
https://doi.org/10.2478/s11686-014-0290-7
-
Alho, M., Schnyder, M., Schaper, R., Meireles, J., Belo, S., Deplazes, P., de Carvalho, L.M. (2016). Seroprevalence of circulating Angiostrongylus vasorum antigen and parasite-specific antibodies in dogs from Portugal. Parasitol Res. 115(7): 2567-2572.
https://doi.org/10.1007/s00436-016-5001-x PMid:27000086 PMCid:PMC4914520
-
Eira, C., Vingada, J., Torres, J., Miquel, J. (2006). The Helminth community of the Red fox, Vulpes vulpes, in Dunas de Mira (Portugal) and its effect on host condition. Wildl Biol Pract. 2, 26-36.
https://doi.org/10.2461/wbp.2006.2.5
-
Doby, J.M., Piedade-Guerreiro, J., Drozdz, J. (1971). Georgraphic distribution of Angiostongylus (Parastrongylus) dujardini Drozdz and Doby, 1970, nematode parasite of small wild rodents. Its appearance in Portugal. An Esc Nacl Saude Publica Med Trop. 5(1): 59-60.
-
Deak, G., Gillis-Germitsch, N., Ionică, A.M., Mara, A., Păstrav, I.R., Cazan, C.D., Ioniță, M., et al. (2019). The first seroepidemiological survey for Angiostrongylus vasorum in domestic dogs from Romania. Parasit Vectors. 12(1): 224.
https://doi.org/10.1186/s13071-019-3481-0 PMid:31088513 PMCid:PMC6515677
-
Deak, G., Gherman, C. M., Ionică, A. M., Vezendan, A. D., D’Amico, G., Matei, I.A., Daskalaki, A.A., et al. (2017). Angiostrongylus vasorum in Romania: an extensive survey in red foxes, Vulpes vulpes. Parasit Vectors. 10(1): 330.
https://doi.org/10.1186/s13071-017-2270-x PMid:28701176 PMCid:PMC5508484
-
Gherman, M., Deak, G., Matei, I.A., Ionică, A.M., D’Amico, G., Taulescu, M., Barbu-Tudoran, L., et al. (2016). A rare cardiopulmonary parasite of the European badger, Meles meles: first description of the larvae, ultrastructure, pathological changes and molecular identification of Angiostrongylus daskalovi Janchev & Genov 1988. Parasit Vectors. 9(1): 423.
https://doi.org/10.1186/s13071-016-1718-8 PMid:27485118 PMCid:PMC4969667
-
Gherman, M., Ionică, A.M., D’Amico, G., Otranto, D., Mihalca, A.D. (2016). Angiostrongylus chabaudi (Biocca, 1957) in wildcat (Felis silvestris silvestris, S) from Romania. Parasitol Res. 115(6): 2511-2517.
https://doi.org/10.1007/s00436-016-5032-3 PMid:27106235
-
Simin, S., Spasojević Kosić, L., Kuruca, L., Pavlović, I., Savović, M., Lalošević, V. (2014). Moving the boundaries to the South-East: first record of autochthonous Angiostrongylus vasorum infection in a dog in Vojvodina province, northern Serbia. Parasit Vectors. 7, 396.
https://doi.org/10.1186/1756-3305-7-396 PMid:25164574 PMCid:PMC4261976
-
Gavrilović, P., Todorović, I., Pavlović, I., Živulj, A. (2018). First report of Angiostrongylus vasorum in red foxes (Vulpes vulpes) in Serbia. J Hellenic Vet Med Soc. 69(4): 1265-1270.
https://doi.org/10.12681/jhvms.19616
-
Gavrilović, P., Marinković, D., Vidanović, D., Dobrosavljević, I., Gavrilović, A. (2019). Are golden jackals (Canis aureus) definitive hosts for Angiostrongylus vasorum? Transbound Emerg Dis. 66(6): 2305-2310.
https://doi.org/10.1111/tbed.13284 PMid:31254445
-
Carretón, E., Morchón, R., Falcón-Cordón, Y., Matos, J., Costa-Rodríguez, N., Montoya-Alonso, J.A. (2020). First epidemiological survey of Angiostrongylus vasorum in domestic dogs from Spain. Parasit Vectors. 13, 306.
https://doi.org/10.1186/s13071-020-04180-5 PMid:32532325 PMCid:PMC7291642
-
Martínez-Rondán, J., de Ybáñez, M.R.R., López-Beceiro, A.M., Fidalgo, L.E., Berriatua, E., Lahat, L., Sacristán, I., et al. (2019). Cardiopulmonary nematode infections in wild canids: does the key lie on host-prey-parasite evolution? Res Vet Sci. 126, 51-58.
https://doi.org/10.1016/j.rvsc.2019.08.008 PMid:31437776
-
Torres, J., Miquel, J., Motjé, M. (2001). Helminth parasites of the eurasian badger (Meles meles ) in Spain: a biogeographic approach. Parasitol Res. 87(4): 259-263.
https://doi.org/10.1007/s004360000316 PMid:11355672
-
-
Alvarez, F., Iglesias, R., Bos, J., Rey, J., Sanmartin Durán, L. (1991). Lung and hearth nematodes in some Spanish mammals. Wiad Parazytol. 37(4): 481-490.
-
Delgado-Serra, S., Sola, J., Negre, N., Paredes-Esquivel, C. (2022). Angiostrongylus cantonensis nematode invasion pathway, Mallorca, Spain. Emerg Infect Dis. 28(6): 1163-1169.
https://doi.org/10.3201/eid2806.212344 PMid:35608603 PMCid:PMC9155863
-
Grandi, G., Lind, E.O., Schaper, R., Ågren, E., Schnyder, M. (2017) Canine angiostrongylosis in Sweden: a nationwide seroepidemiological survey by enzyme-linked immunosorbent assays and a summary of five-year diagnostic activity (2011-2015). Acta Vet Scand. 59(1): 85.
https://doi.org/10.1186/s13028-017-0351-7 PMid:29258532 PMCid:PMC5735942
-
Lurati, L., Deplazes, P., Hegglin, D., Schnyder, M. (2015). Seroepidemiological survey and spatial analysis of the occurrence of Angiostrongylus vasorum in Swiss dogs in relation to biogeographic aspects. Vet Parasitol. 212(3-4): 219-226.
https://doi.org/10.1016/j.vetpar.2015.08.017 PMid:26342624
-
Gillis-Germitsch, N., Manser, M. B., Hilbe, M., Schnyder, M. (2017). Meerkats (Suricata suricatta), a new definitive host of the canid nematode Angiostrongylus vasorum. Int J Parasitol Parasites Wildl. 6(3): 349-353.
https://doi.org/10.1016/j.ijppaw.2017.10.002 PMid:29379713 PMCid:PMC5779638
-
Varodi, I., Malega, A.M., Kuzmin, Y., Kornyushin, V.V. (2017). Helminths of wild predatory mammals of ukraine. nematodes. Vestnik Zoologii. 51(3): 187-202.
https://doi.org/10.1515/vzoo-2017-0026
-
Schnyder, M., Schaper, , Bilbrough, G., Morgan, E.R., Deplazes, P. (2013). Seroepidemiological survey for canine angiostrongylosis in dogs from Germany and the UK using combined detection of Angiostrongylus vasorum antigen and specific antibodies. Parasitol. 140(11): 1442-1450.
https://doi.org/10.1017/S0031182013001091 PMid:23965824 PMCid:PMC3762218
-
Taylor, C.S., Gato, R.G., Learmount, J., Aziz, N.A., Montgomery, C., Rose, H., Coulthwaite, C.L., et al. (2015). Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitol. 142(9): 1190-1195.
https://doi.org/10.1017/S0031182015000463 PMid:26027539
-
Anderson, R.C. (1987). Keys to genera of the superfamily In: D.I. Gibson, Jones, R.A. Bray (Eds.), Keys to the nematode parasites of vertebrates: archival volume (40). CABI Publishing
-
Ubelaker, J.E. (1986). Systematics of species referred to the genus Angiostrongylus. J Parasitol. 72(2): 237-244.
https://doi.org/10.2307/3281599 PMid:3525794
-
-
-
Costa, O., De Araujo Costa, H.M., Guimaraes, M.P. (2003). Redescription of Angiostrongylus vasorum (Baillet, 1866) and systematic revision of species assigned to the genera Angiostrongylus Kamensky, 1905 and Angiocaulus Schulz, 1951. Revue Méd Vét. 154(1): 9-16.
-
Souza, G., Simões, R.O., Thiengo, S.A., Lima, W.S., Mota, E.M., Rodrigues-Silva, R., Lanfredi, R.M., Maldonado Jr, A. (2009). A new metastrongilid species (Nematoda: Metastrongylidae): A lungworm from Akodon montensis (Rodentia: Sigmodontinae) in Brazil. J Parasitol. 95(6): 1507-1511.
https://doi.org/10.1645/GE-2013.1 PMid:19566346
-
del Rosario Robles, M., Navone, G.T., Kinsella, J.M. (2008). A new angiostrongylid (Nematoda) species from the pulmonary arteries of Akodon azarae (Rodentia: Cricetidae) in Argentina. J Parasitol. 94(2): 515-519.
https://doi.org/10.1645/GE-1340.1 PMid:18564753
-
Vieira, M., Muniz-Pereira, L.C., de Souza Lima, S., Neto, A.H., Guimarães, E.V., Luque, J.L. (2013). A new metastrongyloidean species (Nematoda) parasitizing pulmonary arteries of Puma (Herpailurus) yagouaroundi (É. Geoffroy, 1803) (Carnivora: Felidae) from Brazil. J Parasitol. 99(2): 327-331.
https://doi.org/10.1645/GE-3171.1 PMid:23016945
-
Vieira, M., Muniz-Pereira, L.C., Souza-Lima, S.D., Rocha, B.M., Luque, J.L. (2017). Parasitic nematodes of three species of wild carnivore mammals from Atlantic forest in the state of Minas Gerais, Brazil. Rev Mex Biodiv. 88(4): 801-806.
https://doi.org/10.1016/j.rmb.2017.10.033
-
Almeida, R.D., Souza, J.G.R.D., Santos, H.A., Torres, E.J.L., Vilela, R.D.V., Cruz, O. M.S., Rodrigues, L., et al. (2020). Angiostrongylus minasensis n. sp.: new species found parasitizing coatis (Nasua nasua) in an urban protected area in Brazil. Rev Bras Parasitol Vet. 29(1): 018119.
https://doi.org/10.1590/s1984-29612019103 PMid:32049148
-
-
Buckingham, J., Ashby, B. (2022). Coevolutionary theory of hosts and parasites. J Evol Biol. 35(2): 205-224.
https://doi.org/10.1111/jeb.13981 PMid:35030276 PMCid:PMC9305583
-
Jefferies, R., Shaw, S.E., Viney, M., Morgan, E.R. (2009). Angiostrongylus vasorum from South America and Europe represent distinct Parasitol. 136(1): 107-115.
https://doi.org/10.1017/S0031182008005258 PMid:19126274
-
Jefferies, R., Shaw, S.E., Willesen, J.L., Viney, M., Morgan, R. (2010). Elucidating the spread of the emerging canid nematode Angiostrongylus vasorum between Palaearctic and Nearctic ecozones. Infect Genet Evol. 10(4): 561-568.
https://doi.org/10.1016/j.meegid.2010.01.013 PMid:20139034
-
-
Lange, K., Penagos-Tabares, F., Vélez, J., Gutiérrez, J., Hirzmann, J., Chaparro-Gutiérrez, JJ, Piedrahita, D., et al. (2018). Regional report on Angiostrongylus vasorum in Colombia: Genetic similarity to European lineage. Vet Parasitol Reg Stud Reports. 13, 21-23. https://doi.org/10.1016/j.vprsr.2018.03.004 PMid:31014876
-
Mejías-Alpízar, J., Porras-Silesky, C., Rodríguez, EJ, Quesada, J., Alfaro-Segura, MP, Robleto-Quesada, J., Gutiérrez, R., Rojas, A. (2024). Mitochondrial and ribosomal markers in the identification of nematodes of clinical and veterinary importance. Parasite Vectors. 17, 77. https://doi.org/10.1186/s13071-023-06113-4 PMid:38378676 PMCid:PMC10880205
-
Blanch-Lázaro, B., Mitton, Z., Tudor, C., Hindle, J., Martineau, H., Fox, M., Blake, DP (2018). Genetic diversity and population structure of Angiostrongylus vasorum parasites within and between local urban foxes (Vulpes Vulpes). Vet Parasitol. 262, 42-46. https://doi.org/10.1016/j.vetpar.2018.09.008 PMid:30389010
-
Tayyrov, A., Schnetzler, M., Gillis-Germitsch, N., Schnyder, (2021). Genetic diversity of the cardiopulmonary canid nematode Angiostrongylus vasorum within and between rural and urban fox populations. Infect Genet Evol. 87, 104618. https://doi.org/10.1016/j.meegid.2020.104618 PMid:33188914
-
-

 10.2478/macvetrev-2025-0029
10.2478/macvetrev-2025-0029