INTRODUCTIONReptiles inhabit nearly every part of the globe. As poikilothermic animals, they thrive in warm regions such as Asia, Africa, and South America. These animals exhibit remarkable adaptability, occupying diverse ecosystems ranging from tropical forests to deserts (
1). In veterinary medicine, studies on the Iguanidae family, particularly the green iguana (Iguana iguana), are prominent due to their popularity as companion animals in recent decades (
2,
3,
4). However, anthropogenic pressures have led to the endangerment of several iguana species, including the rhinoceros iguana (
Cyclura cornuta cornuta), which is classified as “endangered” on the International Union for Conservation of Nature Red List. This species is named after the modified horn-like scales located dorsally to the snout: two rostral scales behind the nasal openings and a more pronounced medial scale located caudally in the interorbital region. These features are more pronounced in males than in females. Until 2000, there were three subspecies of rhinoceros iguana:
Cyclura cornuta cornuta from the Dominican Republic and Haiti,
Cyclura cornuta stejnegeri from Mona Island, Puerto Rico, and
Cyclura cornuta onchiopsis from Navassa Island, the latter now extinct (
5).
The existing literature on the rhinoceros iguana mainly addresses phylogenetic studies (
6), conservation efforts (
7), and certain clinical conditions (
8,
9). However, detailed anatomical description of the skull remains scarce, despite their relevance for clinical, educational, and conservation applications (
10,
11). In contrast, other iguanid species, such as the green iguana (
Iguana iguana) have been studied more extensively, using traditional and advanced imaging techniques (
12,
13,
14).
Advanced imaging techniques allow better visualization due to the acquisition of multiplanar reconstructions (MPR) and three-dimensional reconstructions, providing valuable information on the structures under study. Common visualization modes include Maximum Intensity Projection (MIP) and Volume Rendering (VR3D) (
15). MIP displays the maximum intensity of pixels along a projection beam in a two-dimensional image, highlighting bone structures and regions of high contrast. Conversely, VR3D creates a three-dimensional image in which the voxels can be programmed for visibility, color, and opacity (
16). The volumetric data can be resampled using computer algorithms and manually edited to distinguish the region of interest from surrounding structures.
Despite the potential of these technologies, no prior study has simultaneously applied multiple CT-based 3D reconstruction techniques to describe the skull of
Cyclura cornuta cornuta. Therefore, this study aimed to evaluate and illustrate the anatomical applicability and fidelity of different 3D reconstruction modalities using
C. cornuta as a representative model. Rather than conducting a comparative anatomical study between species, this work aims to assess how different CT-based imaging methods complement each other in visualizing cranial structures. By doing so, it establishes a methodological framework that may be applied in future research and supports clinical and educational practices related to reptile anatomy.
MATERIAL AND METHODSAnimals
Two carcasses of adult female rhinoceros iguanas (
Cyclura cornuta cornuta) were collected from the zoological park “Rancho Texas Lanzarote Park” in Lanzarote, Canary Islands, Spain. One female measured 94 cm and the other 91 cm in length from snout to tail, weighing 5.0 kg and 4.2 kg, respectively. Both animals died of natural causes, and no abnormalities were found upon physical examination.
Computed tomography study
Computed tomography (CT) images were acquired using a clinical (human diagnostic) 16-slice helical CT scanner (Toshiba Astelion, Canon Medical Systems, Tokyo, Japan). The animals were positioned in ventral recumbency on the CT table for the procedure. A standard clinical protocol was employed (100 kVp, 80 mA, 512×512 acquisition matrix, 1809×858 field of view, spiral pitch factor of 0.94, and gantry rotation time of 1.5 sec) to obtain sequential transverse CT images 1 mm thick, utilizing both soft tissue and bone/ pulmonary algorithms. No notable CT density or anatomical variations were observed in the reptile’s head used for this study.
We used two window settings to enhance the visualization of head structures: a bone window setting (window width (WW)=1,500; window level (WL)=300) and a soft tissue window setting (WW=350; WL=40). Additionally, multiplanar reconstructed (MPR) images were generated for acquiring three-dimensional datasets, and for producing sectional images to better visualize the skull anatomy. The original data served to produce volume-rendered reconstructed images (VR3D) after manually editing the transverse CT images to exclude soft tissues using standard DICOM 3D format software (OsiriX MD, Geneva, Switzerland). Furthermore, the MPR technique was applied to obtain both multiplanar Volume Rendering (MPR-VR) and Maximum Intensity Projection (MIP) reconstructed images, providing critical information on the bony structures. In addition, different drawings were added by using MyEdit.online (CyberLink Corp. Taiwan) to better illustrate all the anatomic formations described.
Anatomic evaluation
The highest-definition images were selected in rostral, lateral, dorsal, ventral, and caudal views. This study focused on evaluating and describing relevant features of the skull, including the cranial bones, the orbital structures, the jaw apparatus, the neurocranial and basicranial elements. Anatomical structures were identified based on comparative references from other iguanid species and classical reptilian skull atlases (
1,
3,
10,
11,
14,
17,
18). Observations were qualitative, focusing on morphology, relative positioning, and notable species-specific traits.
RESULTSDifferent images were obtained through the application of various tomographic reconstruction techniques.
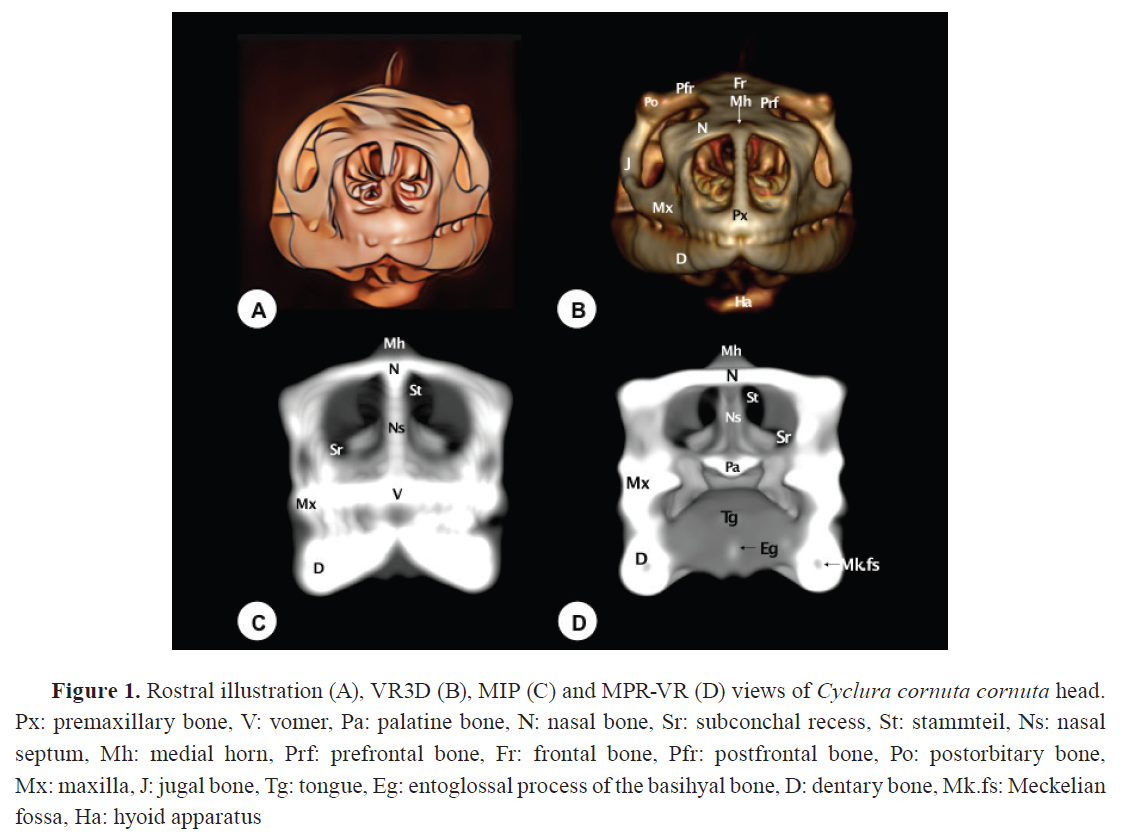
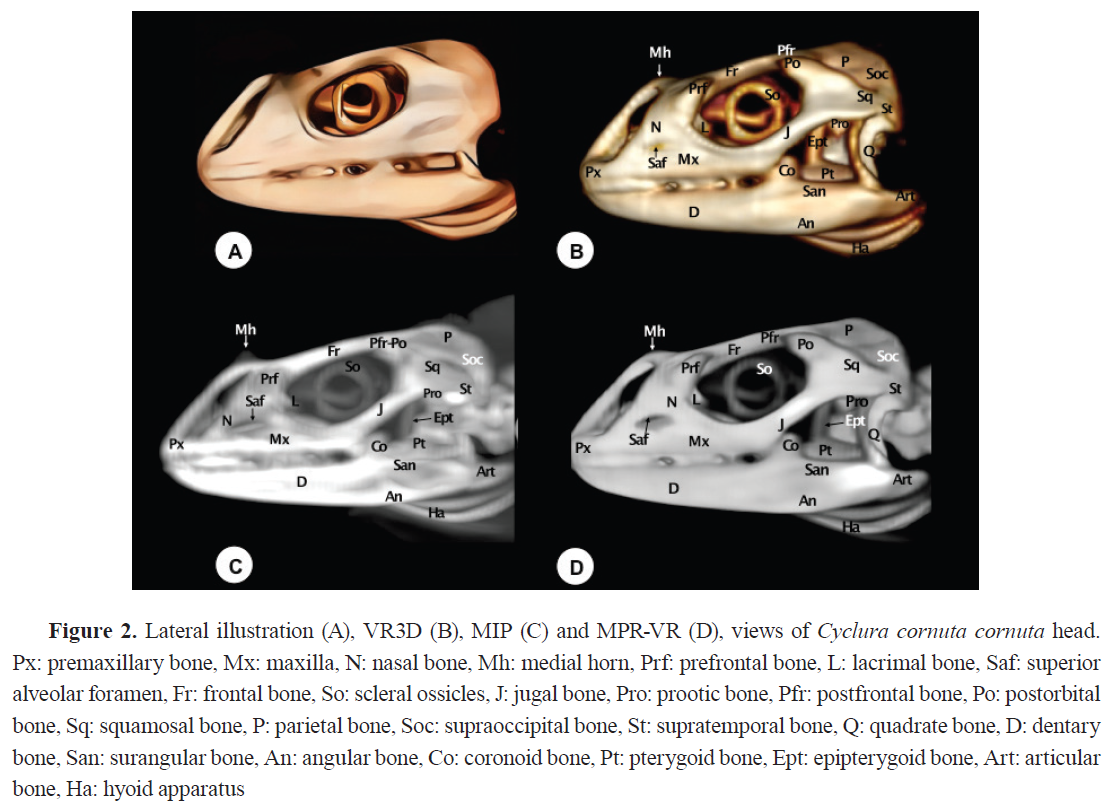
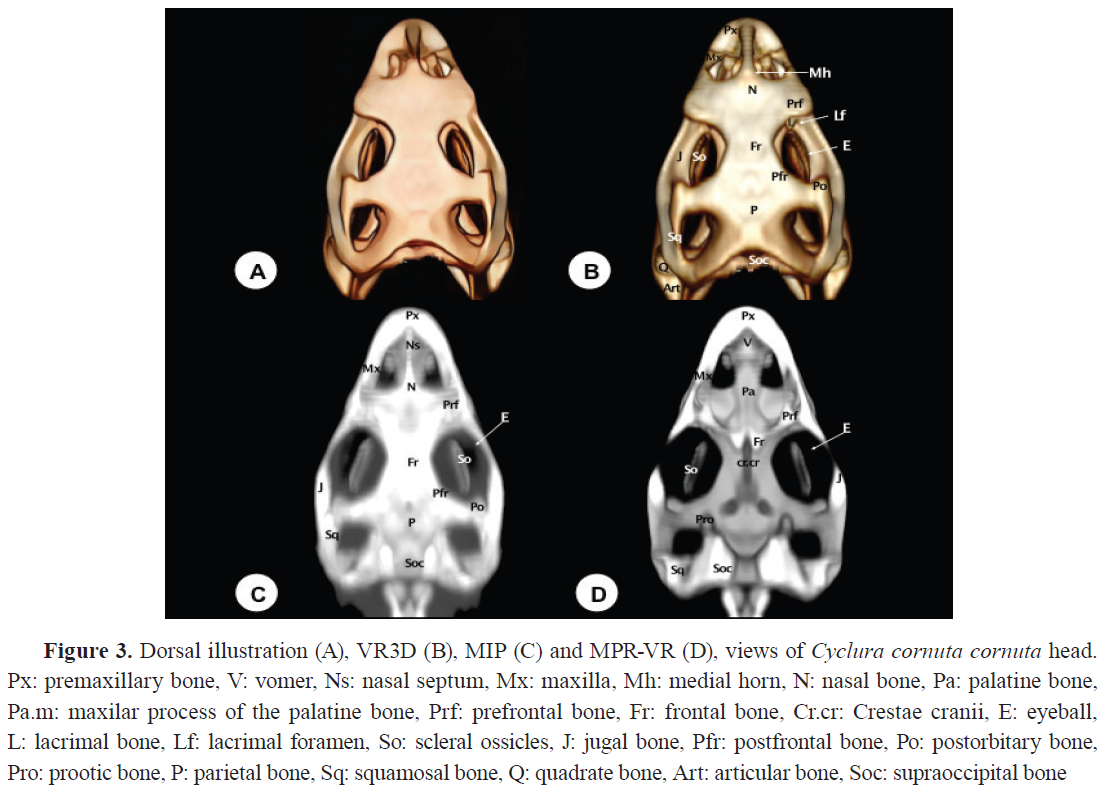
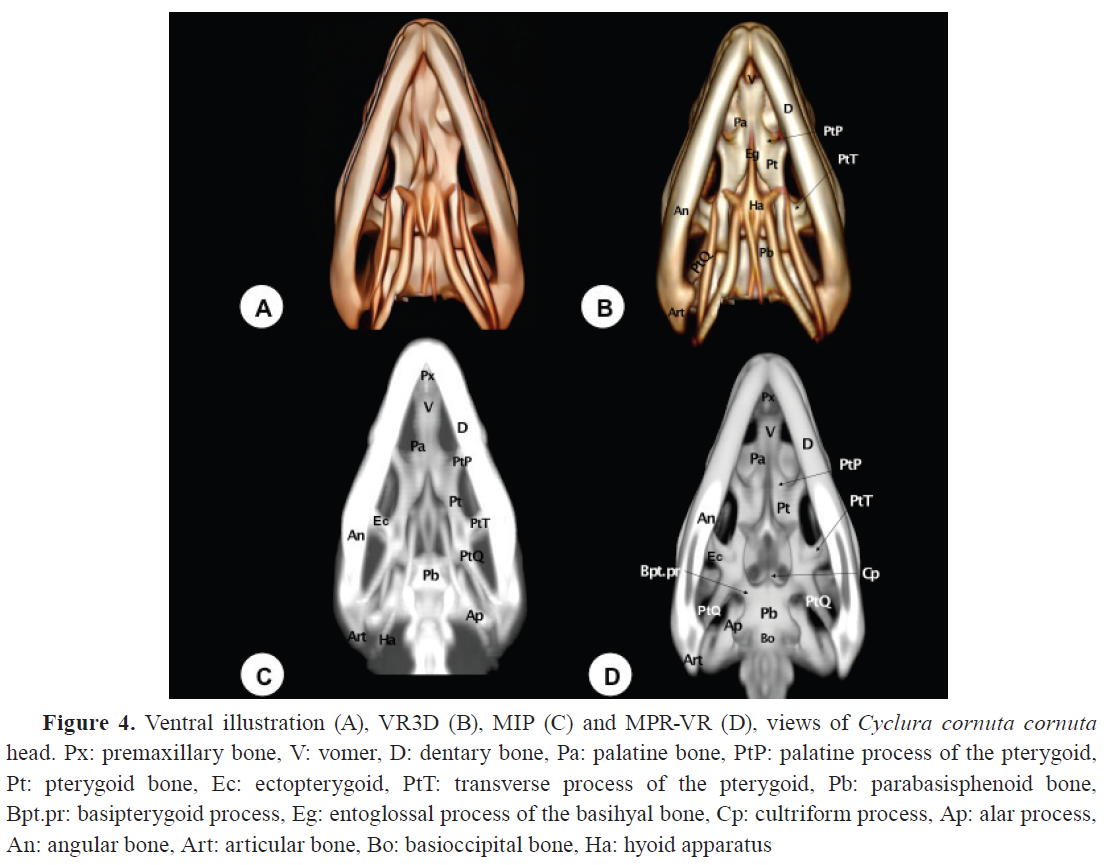
Fig. 1 to
5 consist of: (A) an illustrative drawing, (B) VR3D, (C) MIP, and (D) MPR-VR.
These figures are organized according to anatomical views - rostral (
Fig. 1), lateral (
Fig. 2), dorsal (
Fig. 3), ventral (
Fig. 4), and caudal (
Fig. 5) - to facilitate comprehensive spatial interpretation. Each set of images provided effective visualization of the cranial components of the rhinoceros iguana. The selected images were those that demonstrated the greatest consistency and clarity across techniques, allowing accurate anatomical correlation. The quality and clarity of visualization varied depending on the reconstruction technique applied. Qualitative description of the visibility and interpretability of specific anatomical features are presented in the following sub-sections: A) dermatocranium, B) neurocranium, and C) mandible (illustrated in
Fig. 1,
Fig. 2,
Fig. 3,
Fig. 4,
Fig. 5)
(A) Dermatocranium
Premaxilla (Os premaxillare)
The premaxillary bone exhibited a broad, flat base with a V-shaped morphology (
Fig. 1B,
2,
3). Its articulations with the maxilla, the nasal bone (
Fig. 2,
3D), and the vomer (
Fig. 3D), were distinctly visible in MPR-VR reconstructions. An alveolar plate with 6-7 dentary positions is visible in the rostral three-dimensional image (
Fig. 1B). Compared to conventional axial CT, all 3D reconstructions provided enhanced visualization of the premaxila. However, MPR-VR offered the most precise depiction of its anatomical relationships and internal bone configuration.
Maxilla (Os maxillare)
The maxilla is positioned laterally and ventrally to the nostril on each side of the skull (
Fig. 1,
2, 3). It is a tall and wide bone with a triangular facial process, that articulates anterodorsally with the nasal bone, dorsally with the lacrimal and prefrontal bones, and caudally with the jugal bone (
Fig. 2). It articulates rostrally with the premaxilla through the anterior process, and ventrally with the pterygoid bone through the posterior process, forming the ventrolateral wall of the nasal fossa (
Fig. 3B, C). The maxilla presents an alveolar border with teeth extending to its posterior end, showing no toothless region (
Fig. 2B). An opening on the lateral surface of both maxillary bones corresponds to the superior alveolar canal, through which the maxillary nerve and accompanying blood vessels pass (
Fig. 2). The bone was clearly observed in MPR-VR, where interactive navigation permitted visualization in multiple planes. VR3D illustrated the outer shape effectively, while MIP produced overlapping images that limited interpretation. Axial CT allowed its identification but lacked depth perception.
Nasal bone (Os nasale)
The nasal bone is a paired bone that articulates at the mid-sagittal plane (
Fig. 1,
2,
3B, C). It exhibits a roughly triangular shape, slightly convex, forming the dorsocaudal wall of the nostrils. Both VR and MPR-VR imaging reveal its articulations: rostrally with the premaxillary bone, caudodorsolaterally with the prefrontal bone, lateroventrally with the maxillary bone, and laterocaudally with the lacrimal bone (
Fig. 2). Additionally, this bone displays a protuberance (pseudohorn) with soft tissue attenuation located at the caudal border of the nostrils along the midline of the nasal bone (
Fig. 3B). In contrast, internal nasal structures, including conchae, septum, and associated air spaces, were best visualized with MPR-VR. MIP failed to depict these regions due to masking by adjacent high-density bone. CT axial sections provided fragmented and limited views of these structures, emphasizing the advantage of multiplanar imaging for internal nasal anatomy.
Vomer
The vomer was visualized as a roughly triangular paired bone with its apex directed rostrally (
Fig. 1C,
3D,
4). Located ventromedial to the vomeronasal organ, it serves as its support. It is a toothless structure that joins rostrally with the maxillary and premaxillary bones, caudally with the palatine bones, and medially with its contralateral (
Fig. 3D,
4). This bone was better depicted with MPR-VR. In contrast, MIP provided poor definition in this area due to overlapping density artifacts. CT sections gave poor depiction of vomer disposition, reinforcing the need for multiplanar and volume-enhanced techniques.
Palatine bones (Os palatinum)
The palatine bones, located dorsal to the choana, form part of the medial wall of the suborbital foramen and are toothless. The MPR-VR allowed dynamic exploration of these bones in horizontal and oblique planes (
Fig. 1D,
3D,
4B-D). They articulate rostrally with the vomer, laterally with the maxillary and prefrontal bones, and caudally with the pterygoid bone (
Fig. 3D). Similarly, as the the vomer, CT sections gave an incomplete picture of palatal complexity compared to the 3D reconstructions.
 Prefrontal bone
Prefrontal bone
The prefrontal bone is a paired triangular plate located on the posterolateral wall of the nasal capsule. It contacts the nasal bone rostromedially and ventrally with the maxillary bone (
Fig. 1B,
2,
3). It features a dorsal process connecting with the frontal bone (
Fig. 3D) and a ventral process contacting the palatine, jugal, and lacrimal bones (
Fig. 2C).
Frontal (Os frontale) and Parietal (Os parietale) bones
The frontal bone contributes to the dorsal orbital margin (
Fig. 1B,
2,
3). It is an unpaired bone with lateral projections that fit between the nasals and prefrontal bones (
Fig. 2), and a short medial process extending between the nasals at the midline (
Fig. 2C). Caudally, it contacts the parietal bone, and laterally, it is separated from the postorbital bone by the postfrontal bone (
Fig. 3B, 3C). MPR-VR of a dorsal section reveals that the frontal bone has shallow divergent ridges, known as
Cristae cranii, forming a narrow channel for the olfactory tracts (
Fig. 3D). The parietal bone is an unpaired short bone with anterolateral processes that contact the postfrontal bones rostromedially and the postorbital bones laterally (Fig. 3). It also has post- parietal processes that meet the squamosal bones caudolaterally (Fig. 3), the paraoccipital processes caudomedially (
Fig. 5), and the supratemporal bones laterally (
Fig. 2).
The parietal and frontal bones forming the skull roof were clearly rendered in VR3D, which provided a topographically accurate and visually comprehensive representation. MIP accentuated the sutures and contours but frequently caused overlapping and intensification of signal in thicker regions. MPR-VR allowed detailed multiplanar views that clarified the internal shape of the cranial vault, particularly useful for evaluating bone thickness and spatial orientation. In comparison, the CT axial images provided limited spatial context for these flat, curved structures.
Postfrontal bones (Processus postfrontalis)
The postfrontal bones are small, paired bones that delineate the caudodorsal margins of the bony orbits (
Fig. 1B,
2,
3B, C). They articulate rostrally with the frontal bone, laterally with the postorbital bone, and medially with the parietal bone (
Fig. 3B, C). These articulations were displayed with better detail through MIP and MPR-VR reconstructions.
Postorbital bone (Processus postorbitalis)
The postorbital bone is a roughly triangular paired bone, whose dorsal apex joins the postfrontal bone to form the posterior margin of the orbit (
Fig. 1B,
2,
3B, C,
5). It contacts dorsocaudally with the parietal bone, ventrally with the jugal bone, and caudolaterally with the squamosal bone (
Fig. 3B, C).
Lacrimal bone (Os lacrimale)
The lacrimal bone is a small-paired bone located at the orbital margin (
Fig. 2,
3). It contacts the ventral process of the prefrontal bone, the maxillary bone, and the jugal bone (
Fig. 2B-D). The lacrimal foramen, through which the nasolacrimal duct passes, is slightly visible thanks to VR3D postprocessing (
Fig. 3B). This reconstruction procedure provides excellent depiction of this bone compared to the rest of these techniques.
Jugal bone (Os jugale)
The jugal bone runs along the infraorbital edge of the maxilla, forming the lateral border of the bony orbit (
Fig. 1B,
2,
3). Its medial portion is long and broad, while its caudal portion is curved and thin. Rostrally, it contacts the prefrontal and lacrimal bones (
Fig. 2C) and rostroventrally with the maxilla (
Fig. 2). Dorsally, it articulates with the postorbital and squamosal bones, enclosing the caudolateral margin of the orbit (
Fig. 2). These reconstruction procedures allow relevant delineation of the bone, although the VR3D offered superior contrast (
Fig. 2B,
3B).
Squamosal bone (Os squamosum)
The squamosal bone is a small-paired bone on the lateral surface of the otic capsule (
Fig. 2,
3,
5). It has a triradiate morphology with three processes: an anterior process contacting the postorbital and jugal bones, a posterior process contacting the parietal and supratemporal bones, and a ventral process contacting the dorsal edge of the quadrate bone (
Fig. 2B-D,
5B-D). This bone was well visualized in VR3D, where its external contour and contribution to the supratemporal arch were readily appreciated. MIP and MPR-VR reconstructions allowed for more precise delineation of its articulations, as well as its extension along the temporal fossa. Compared to axial CT, which displayed the squamosal only in partial sections, the combined use of reconstruction techniques offered superior anatomical resolution and contextual integration of this element within the cranial framework.
Quadrate bone (Os ouadratum)
The quadrate bone comprises a central pillar supporting two condyles: the dorsal (cephalic) and ventral (mandibular) condyles. The cephalic condyle is rounded and joins the paroccipital process laterocaudally and the supratemporal bone dorsomedially (
Fig. 2,
3B). The mandibular condyle divides into the medial wing, which articulates with the pterygoid bone, and the lateral wing, whose elevated edge (tympanic crest) supports the tympanum (
Fig. 5B, D). This bone was identifiable in all reconstructions but with differing levels of detail. VR3D allowed external viewing of its articulation with the lower jaw, while MPR-VR offered superior visualization of its internal morphology and spatial position between the mandible and braincase. MIP provided a highly contrasted but sometimes distorted outline. Compared to axial CT, multiplanar reconstructions gave more coherent anatomical relationships of this key bone.
Pterygoid bones (Os pterygoideum)
The pterygoid bones, paired structures, form the bony wall of the caudal palate and the caudomedial wall of the suborbital foramen (
Fig. 2,
4,
5). They present palatine (anterior) and quadrate (posterior) processes. The palatine processes join the palatine bones rostrally, while the quadrate processes articulate with the basipterygoid processes and the medial wing of the quadrate bone caudomedially (
Fig. 5B, D). They also join the epipterygoid bone via a synovial joint dorsally and the ectopterygoid bone laterally (
Fig. 2,
5B, D). These joints were delineated adequately using the MPR-VR reconstruction technique, which were otherwise challenging to evaluate using unprocessed CT data alone.
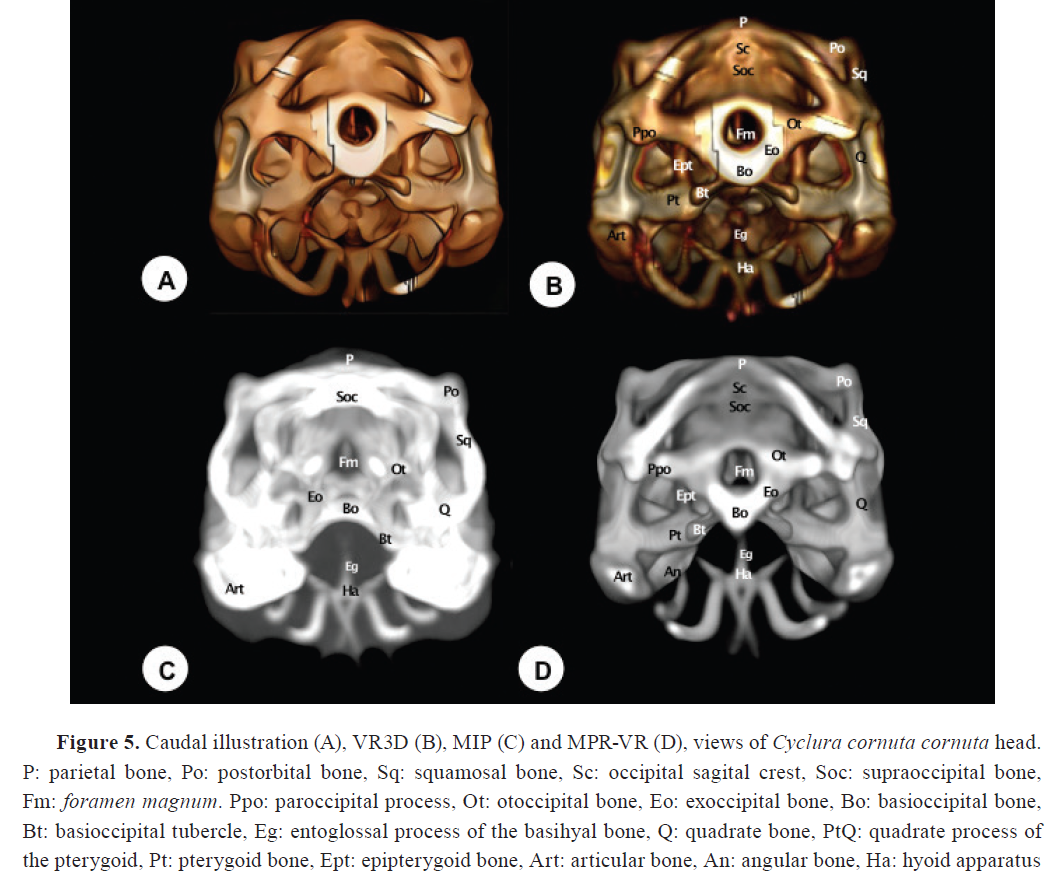 (B) Neurocranium
(B) Neurocranium
Parabasisphenoid bone (Os sphenoidale)
The parabasisphenoid is a trapezoidal bone forming the floor of the cranial cavity. It articulates with the pterygoid rostrolaterally and the basioccipital caudally (
Fig. 4). On its rostral surface, two lateral processes, known as the basipterygoid processes, are short and thick, articulating with the pterygoid bone (
Fig. 4D). Between these processes, a pointed structure termed the
Rostrum parasphenoidale (cultriform process) is observed (
Fig. 4D). Additionally, on its caudal surface, two alar processes are present, distinguishable in the MPR-VR image (
Fig. 4D).
Basioccipital bone (Os basioccipitale)
The basioccipital bone is approximately rectangular and located in the posterior half of the ventral plane (
Fig. 4D,
5). It forms the caudal floor of the skull and the medial portion of the occipital condyles. Caudally, it has two lateral elevated facets that contact the exoccipital bone. Rostrolaterally, it articulates with the prootic bone and the parabasisphenoid bone (
Fig. 4D).
Supraoccipital (Os supraoccipitale)
The supraoccipital bone forms the dorsal part of the
Foramen magnum (
Fig. 2,
3,
5). It articulates dorsally with the parietal bone, ventrally with the exoccipital bone, and rostrally with the prootic bone (
Fig. 5). The dorsal surface features a low ridge known as the sagittal crest (
Fig. 5B,
D). Laterally, the bone expands to enclose the upper part of the braincase.
Exoccipital bone (Os exoccipitale)
The exoccipital bone is a relatively small, thick, and triangular structure that forms part of the caudal wall of the skull, bordering the caudolateral edges of the
Foramen magnum. It articulates caudomedially with the basioccipital and dorsally with the otoccipital bone (
Fig. 5).
Otoccipital and Prootic bones
The otoccipital bone is located ventrolaterally to the supraoccipital and dorsolaterally to the basioccipital bone. It forms part of the lateral portion of the
Foramen magnum, and was well depicted with all the reconstructed images (
Fig. 5B-D). Concerning the prootic bone, it constitutes the rostroventral third of the otic capsule, forming part of the cochlear cavity as well as the roof and lateral wall of the tympanic cavity (
Fig. 2B-D,
3D).
Laterally, it contacts the paraoccipital processes, dorsally the supraoccipital bone (
Fig. 3D), and ventrally the parabasiesphenoid bone. Due to its location and overlap with adjacent structures, visualization of this bone was only possible through VR3D and MPR-VR views (
Fig. 2B,
3D).
(C) Mandible
Dentary bone (Os dentale)
The dentary bone is the principal bone of the mandible (
Fig. 1,
2,
3). It contacts the coronoid, surangular, and angular bones caudally (
Fig. 2). Medially, it features an alveolar platform with 20-25 tooth positions (
Fig. 2C). Rostrally, it tapers to form a rounded symphyseal surface (
Fig. 1B). Relevant internal structures, including the Meckellian fossae were clearly observed in the rostral MPR-VR image (
Fig. 1D). VR3D rendered the external surfaces effectively, useful for spatial orientation. MIP showed the overall contours but often exaggerated the density and obscured fine details, particularly at the level of the tooth roots. Compared to axial CT, which could only show segmented views, the 3D reconstructions greatly enhanced interpretation of jaw anatomy.
Coronoid bone (Os coronoideum)
The coronoid is a triangular bone located dorsally on the labial aspect, between the dentary and surangular bones (
Fig. 2). It has five processes: dorsal, labial, anteromedial, posteromedial, and posterior. The dorsal process is thin and conical, betterdistinguishableinthelateralimage(
Fig. 2B-D). In VR3D, it was partially obscured by adjacent mandibular structures, while in MIP it was poorly defined due to overlapping bone density.
Angular (Os angulare) and Surangular (Os surangulare) bones
The angular bone is a thin, lamellar structure extending along the medial aspect of the mandible, whereas the surangular bone forms the dorsocaudal aspect of the mandible, lying caudal to the coronoid bone. These bones were consistently identified with all the reconstructed procedures (
Fig. 2B-D,
4B-D). Rostrally, they contact the dentary bone, and caudally, the articular bone.
Articular bone (Os articulare)
The articular bone is located on the dorsocaudal surface of the mandible. It articulates rostrally with the surangular bones. On its dorsal aspect, it presents condyles that contact the quadrate bone (
Fig. 2B-D,
3B,
4B-D,
5B-D). CT could only provide limited and segmented views of the articular bone, whereas the 3D reconstructions greatly enhanced the interpretation of its morphology, spatial orientation, and articulations with adjacent mandibular structures.
Hyoid apparatus (Os hyoideum)
The hyoid apparatus is displayed in the intermandibular space (
Fig. 1B,
2B-D,
4B, C,
5B-D). This structure supports the tongue and larynx. It consists of a pentagonal piece (
Fig. 4B) with three components: a central (basihyal) structure, an anterior process, and two caudolateral processes. The anterior process of the hyoid apparatus (the entoglossal process of the basihyal bone) is revealed as a hyperattenuating structure in the rostral MPR-VR image (
Fig. 1D). Compared to axial CT, which provided only fragmented and low-contrast views of the hyoid apparatus, the 3D reconstructions, especially VR3D and MPR-VR, significantly improved the visualization of its delicate elements, their spatial arrangement, and their articulation with the skull base.
DISCUSSION
Advanced imaging techniques have become increasingly valuable in veterinary anatomy, particularly in the study of exotic and endangered species (
11,
12,
13,
14). In this study, we used
Cyclura cornuta cornuta as a model to evaluate three CT-based post-processing techniques with the aim of identifying their respective strengths and limitations in visualizing cranial structures.
Our outcomes demonstrated that these reconstruction techniques allowed us to identify the cranial bones and to document several unique features that distinguish this species from other iguanids, such as the green iguana (
Iguana iguana), which is considered the reference in the family Iguanidae (
1,
2). Therefore, the different projections served to differentiate the triangular morphology that characterizes the skulls of these animals (
17). Besides, lateral views revealed an elongated preorbital region in rhinoceros iguanas compared to green iguanas (
10,
11). This difference could be related to functional adaptations in the feeding and respiratory process by facilitating the arrangement of nasal and maxillary structures (
17).
The VR3D technique offered a comprehensive and intuitive three-dimensional representation of the skull, allowing for an overall spatial understanding of anatomical relationships. It proved especially useful for educational and illustrative purposes, as it provides a realistic, life-like rendering. However, some deeper or less mineralized structures were less sharply defined in this mode, and overlapping densities occasionally limited precise differentiation between adjacent bones. Therefore, VR3D allowed the observation of the pointed and long snout, as well as the dorsal modification of the nasal bone, including a small bony prominent tubercle that resembles a horn. This structure was better visualized in the MIP reconstructions, showing a triangular shape with soft tissue attenuation. Thus, MIP emphasized highly radiopaque areas by displaying the voxels with maximum intensity along each ray projection. This technique was particularly effective for highlighting bony prominences and delineating dense structures such as the rostral protuberances (horns), which are absent in other iguanids, including the green iguana or the marine iguana (
Amblyrhynchus cristatus), in which the snout is modified and shortened (
17,
18), and the dorsal surface is gently rugose and pierced by several foramina (
18). However, due to its nature, MIP often resulted in loss of internal anatomical detail and could create visual artifacts or misleading impressions of bone thickness, especially in overlapping regions.
MPR-VR, which combines multiplanar views with interactive 3D visualization, allowed precise identification of both surface and internal anatomical landmarks. It enabled cross-sectional exploration while preserving spatial orientation, making it the most versatile technique for anatomical analysis. This method facilitated correlation between axial, sagittal, and coronal planes, which is particularly beneficial in clinical contexts for diagnostic imaging. Hence, this procedure allowed the identification of the cultriform process that was less developed in the rhinoceros iguana than in other iguanid species, such as the green iguana (
17), appearing as a pointed structure with its tip pointing rostrally. Similar findings have been identified in the marine iguana, where this process is also quite short, showing an overall triangular shape (
18). This little development may be related to functional differences in soft tissue support of the palate and pharynx, as well as muscular insertions associated with swallowing (
17). The ventral views of the different reconstruction techniques allowed the observations of well-defined broad processes articulating with the quadrate and the palatine bones. These processes were quite robust in
C. cornuta, possibly in response to the mechanical demands of chewing fibrous plant material, its main food source. Moreover, this reconstruction technique revealed a well-defined channel in the cranial crest for the passage of part of the olfactory system. This organization has been reported in other studies (
10), suggesting, a greater adaptive olfactory sensitivity to its arid island environment.
It is important to highlight that the proximity of bones with similar densities made it difficult to differentiate accurately three-dimensional reconstructed images. Thus, subjective image analysis and objective measurements revealed a relatively small hyoid apparatus in the rhinoceros iguana compared to the green iguana (
17). However, we could not distinguish the different components of the hyoid, which could be due to the superposition and its low density. Despite this, MIP and MPR VR reconstructions were essential to identify critical sutures and interosseous relationships, providing anatomical detail not achievable with conventional techniques. Despite the benefits of these reconstructions, when comparing the three modalities, it became evident that no single method was sufficient on its own to fully depict the complexity of the cranial anatomy. VR3D excels in spatial context, MIP in contrast enhancement of dense structures being critical for providing detailed and accurate information, and MPR-VR in detailed and plane-specific visualization (
15).
Therefore, the combination of different CT reconstruction techniques (VR3D, MIP, MPR-VR) was pivotal for the anatomical description of the rhinoceros iguana skull, useful for clinical purposes detecting fractures, abscesses, and metabolic bone disease in iguanas in rescue programs and zoos and as a teaching tool of comparative anatomy in veterinary schools (
19).
Although this study focused solely on
Cyclura cornuta cornuta, the methodological insights can be extrapolated to other reptilian species, especially those requiring non-invasive anatomical evaluation. The high-resolution images generated here may also serve as a reference for comparative morphology in future studies which can provide new species-specific data.
CONCLUSION
This study evaluated the anatomical utility of three CT-based 3D reconstruction techniques using two specimens of
Cyclura cornuta cornuta as a representative model. Each method contributed complementary insights into the cranial anatomy, highlighting different bone structures with varying degrees of clarity and spatial context. Rather than comparing species anatomically, our goal was to demonstrate how these imaging modalities can enhance anatomical visualization and understanding in reptiles. The findings support the use of a multimodal CT-based approach as a valuable tool for veterinary diagnostics, anatomical education, and future studies in reptile morphology. Additionally, this work provides a high-resolution imaging reference for an endangered species, contributing to the anatomical documentation of
Cyclura cornuta cornuta and offering a methodological framework applicable to other exotic or conservation-relevant reptiles.
CONFLICT OF INTEREST
The authors declare that they have no financial or non-financial conflict of interest regarding authorship and publication of this article.
ACKNOWLEDGMENTS
In loving memory of Mr. Álvaro Domingo and Mr. Honorio Rodríguez García, both formerly affiliated with El Cairo Trade Center. We extend our heartfelt thanks to Mr. Ayesh Mohamad, Mrs. Nicolasa Rodríguez, Mrs. Carmen Mingot, Mrs. Emilia Mingot, Mrs. Concepción Mingot, Mr. Nicolás Aquino, Mrs. Marisa Mohamad, and Mr. Jamal Jaber, all members of El Cairo Trade Center, for their support and constructive comments. We are also grateful to Miss Sara Carrascosa (independent illustrator) for her valuable assistance with the figures, and to Rancho Texas Lanzarote Park (Lanzarote, Canary Islands, Spain) for kindly providing the animals used in this study.
AUTHORS’ CONTRIBUTIONS
JRJ made the conceptualization. JRJ, AM-E, FS-C and ME conducted the methodology. JRJ performed the formal analysis. JRJ, FS-C, and AM-E were involved in the investigation. ME was responsible for resources. JRJ wrote and prepared the original draft. JRJ, FS-C, AM-E and ME were included in writing-review and editing, All authors have read and agreed to the published version of the manuscript.

 10.2478/macvetrev-2025-0030
10.2478/macvetrev-2025-0030




