INTRODUCTION
Ovarian cancer is one of the most frequent malignancy in gynecology, affecting one or both ovaries with a high mortality rate (
1,
2). Ovarian cancer may manifest in various malignant tumors in the peritoneal cavity (
3,
4). The three main categories of ovarian tumours are epithelial, which is the most common, germ cell, and sex-cord-stromal tumours.
The latter two represent approximately 5% of all ovarian cancers. The histological sub-variants of epithelial ovarian cancer include endometrioid, mucinous, serous, and clear cells. Endometrioid, mucinous, and clear cell subtypes make up 10%, 3%, and 10%, respectively. Serous tumors account for 70–80% of all cases (
5).
Because of its uncertain pathophysiology, several hypotheses have been proposed to explain the development of ovarian carcinoma. The “Incessant ovulation theory” proposes an inflammatory process involving leukocyte infiltration, inflammatory mediators, and reactive oxygen species production that can damage DNA. The “Gonadotropin hypothesis” proposes that an excessive gonadotropin aids the development of ovarian cancer by acting directly or indirectly through amplified estrogen production. The “Hormone stimulation hypothesis” proposes that excessive androgen or estrogen stimulation of the ovarian surface epithelium (OSE) endorses neoplastic transformation (
6).
The major factors that contribute to the progress of ovarian tumor are age, genetics, infertility, postmenopausal hormone treatment, and nulliparity (
7). Obesity is considered to be associated with multiple malignancies, including colorectal, endometrial, and breast carcinoma. Currently, there are around 1.5 million cases with ovarian cancer and half a million cancer deaths per year, with roughly one in five obesity cases (
8,
9). The condition of obesity is linked to modifications in lipid profiles, characterized by heightened levels of total cholesterol, circulating low-density lipoprotein (LDL), and triglycerides (TG), as well as decreased levels of high-density lipoprotein (HDL) and adiponectin. Such changes may contribute to the onset of ovarian cancer. Obesity-induced abnormal synthesis of cytokines, hormonal factors, adipokines, and adipose tissue- related inflammatory responses may influence ovarian cancer development. Interestingly, multiple studies have reported low adiponectin plasma levels with ovarian carcinogenesis (
10,
11,
12,
13). Adiponectin plays a crucial role in the energy balance regulation, influencing the metabolism of glucose and lipids through the activation of AMP- activated protein kinase (AMPK) and peroxisome proliferator-activated receptors (PPAR-α). Diminished adiponectin levels may augment the atypical development of ovarian cancer, influenced by the ongoing activation of the PI3K/Akt/mTOR signalling system (
14,
15,
16). As a result, it is plausible to anticipate that a rise in adiponectin levels may benefit traditional ovarian cancer treatments.
Carboplatin is one of the preferred platinum- based drugs for ovarian cancer, acting by blocking of the mTOR-mediated signalling system and arresting the G
0/G
1 phase of the cell cycle, leading to apoptosis and increased sensitivity of ovarian cancer cells (
17,
18).
Pioglitazone, a thiazolidinedione (TZD) class of anti-diabetic drug, activates PPAR-γ to improve insulin sensitivity and normalize blood glucose levels. PPAR-γ ligands have anticancer effects on several neoplastic cells, in addition to their insulin-sensitizing and metabolic properties. It also reduces serum TG and free fatty acid levels while also inhibiting TNF-α and resistin synthesis and release (
19).
Based on this information, this study investigated the chemopreventive efficacy of carboplatin and its combination with pioglitazone in mice with high-fat diet-induced ovarian carcinogenesis, with a specific focus on adiponectin augmentation therapy.
MATERIAL AND METHODS
Materials
The reagents and chemicals employed in this research were of analytical grade. Carboplatin was sourced from Fresenius Kabi Oncology Ltd, located in Gurgaon, India, while pioglitazone was obtained from USV Private Limited in Mumbai, India. Lipid profile analysis kits were purchased from Proton Biologicals India Pvt. Ltd., Bangalore, India. Proinflammatory markers analysing ELISA kits were obtained from Universal Biotechnology, New Delhi, India.
Animals
Thirty colony-bred adult female BALB/c mice (25-35 g) were procured from an authorized breeder and housed under regular environmental conditions with a 12 h light/dark cycle, 23±2 °C room temperature, and 60±10% of relative humidity. Each cage held three mice and was lined with husk bedding. The mice were acclimatized in a laboratory setting one week before the trial. The study protocol and the use of animals were in accordance with CCSEA, New Delhi norms (IAEC approval number: SVCP/IAEC/PG/3/4/2022).
Selection of dose
The clinical dose for carboplatin for ovarian cancer in humans is 10.8 mg/kg (
20), and the pioglitazone is 15 mg/kg and 45 mg/kg (
21). The mouse dose of carboplatin was 133 mg/kg. The pioglitazone doses of 3 mg/kg and 9 mg/kg were considered as low and high doses respectively based on the body surface area (
22).
Experimental design
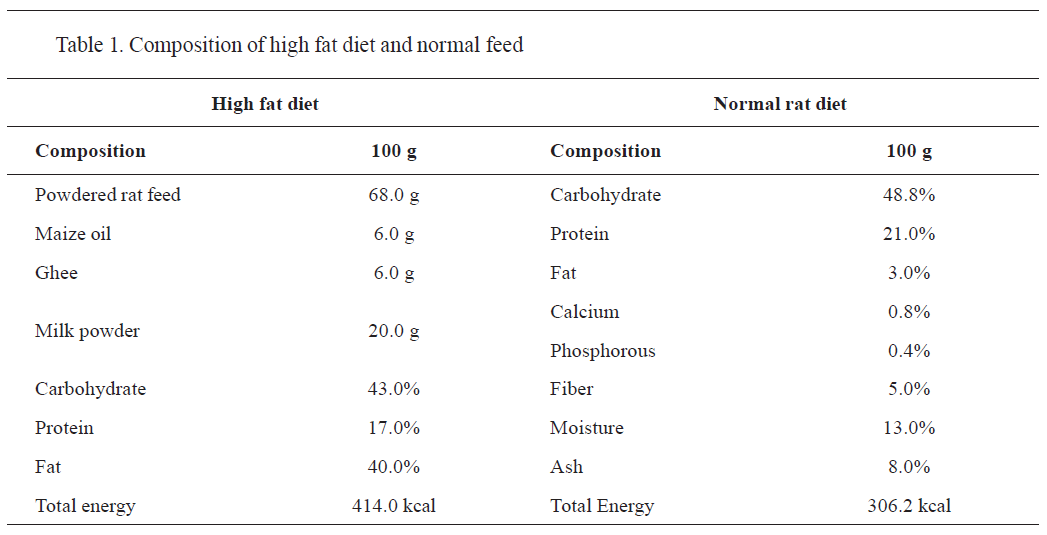
The high-fat diet (HFD) was prepared as described by Abdul Kadir et al. (
23). The composition of HFD (43% carbohydrate, 40% fat, and 17% protein) is shown in
Table 1. Thirty mice were randomly allocated in five groups of six. The prepared high-fat diet was provided for 14 weeks to the entire group except for group I (control), which received a regular standard diet. At 8-week-obesity was confirmed by measuring the serum lipid profile, adiponectin, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and plasma monocyte chemoattractant protein (MCP-1). Starting from week 8, group II was considered as untreated group (positive control), whereas groups III-V were considered as treatment groups. The treatment groups III-V were administered with carboplatin (133 mg/kg i.p.). Groups IV and V were administered with pioglitazone 3 mg/kg and 9 mg/kg p.o., respectively for 6 weeks.
Measurement of body weight and feed intake
The weekly body weight changes and daily feed intake of the individual groups were measured using a standard scale until the end of the trail period. The changes in body weight were calculated based on the initial body weight and final body weight. Based on the daily feed intake, the mean feed intake on day 56 and day 98 was calculated for the feed intake analysis.
Hematological analysis
The blood samples for hematological and serological analysis were taken from the retroorbital plexus on 56
th and 98
th day of the study (
24). The hematological parameters red (blood cells-RBC, hemoglobin-Hb, white blood cells-WBC, and packed cell volume-PCV) were measured with YUMIZEN H500 hematological auto-analyzer. The erythrocyte sedimentation rate (ESR) was measured by the Westergren method.
Serological analysis
On the 56
th and 98
th day of the study, serum total cholesterol (TC), Low-density lipoprotein (LDL), triglycerides (TG), and high-density lipoprotein (HDL) were measured with lipid plus cholesterol monitor VITROS 5.0/FS analyzer. adiponectin, tumor necrosis factor (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein (MCP-1) were analyzed by the ELISA method using standard kits.
Measurement of relative organ weightAfter the 14
th week of the trial period, the mice were euthanized by overdose anesthesia. The organs (spleen, liver, kidney, fallopian tube, and ovary) were dissected and weighed separately with a standard weighing balance. The abdominal fat pad weight was measured with the same method. Further, the relative organ weight (ROW) was calculated based on body weight (
25).
ROW=Organwt./Bodywt.x100
 Measurement of tumor morphology and volume
Measurement of tumor morphology and volume
Ovarian tumor volume was estimated by measuring its length (L) and breadth (W) with a vernier caliper (
26).
Histopathological evaluation
The left ovary of each group was removed immediately after treatments, cleansed of adhering adipose tissues, and weighed. They were preserved in neutral formalin, and then fixed in paraffin wax. Tissue sections of 5 µm were prepared and were stained with hematoxylin and eosin stains. The histopathological alterations of ovary tissue sections were examined by using light microscope at 100x magnification.
Statistical analysis
The data were expressed as mean ± standard deviation (SD) to determine significance and to compare animals across various time points or between groups. A one-way analysis of variance (ANOVA) was performed, accompanied by Tukey’s multiple comparisons, by using the GraphPad statistical software version 8.0. P-values less than 0.05 were regarded as statistically significant.
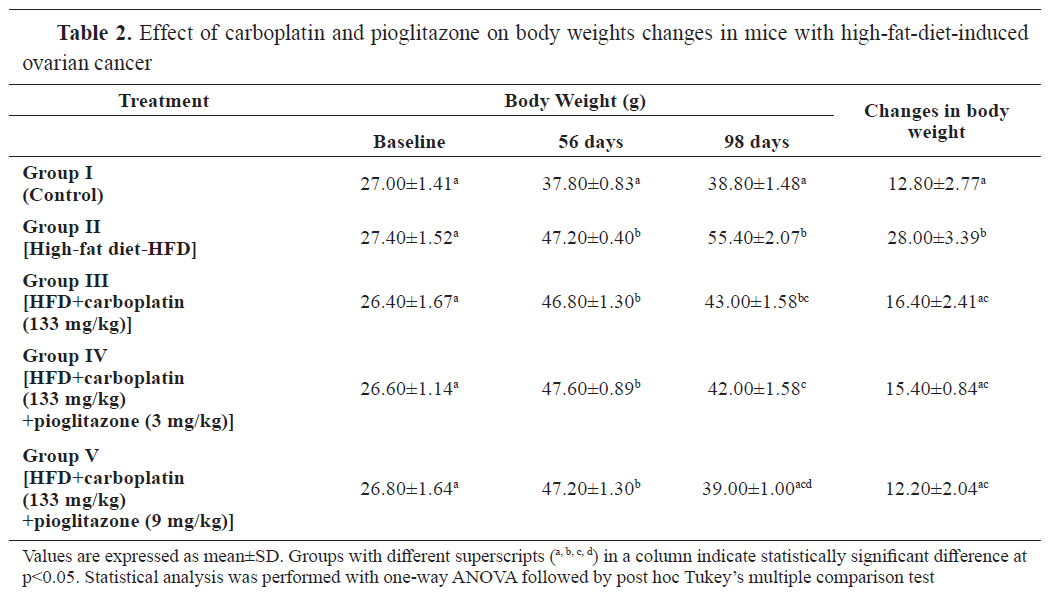
RESULTSThe body weight was significantly higher (p<0.001) in all HFD-fed groups at 56 days compared to the control (group I). Groups II, III, and IV had significantly higher body weights than the control at 98 days. The highest body weight gain (p<0.001) was observed in group II. The other groups were non-significantly different (
Table 2).
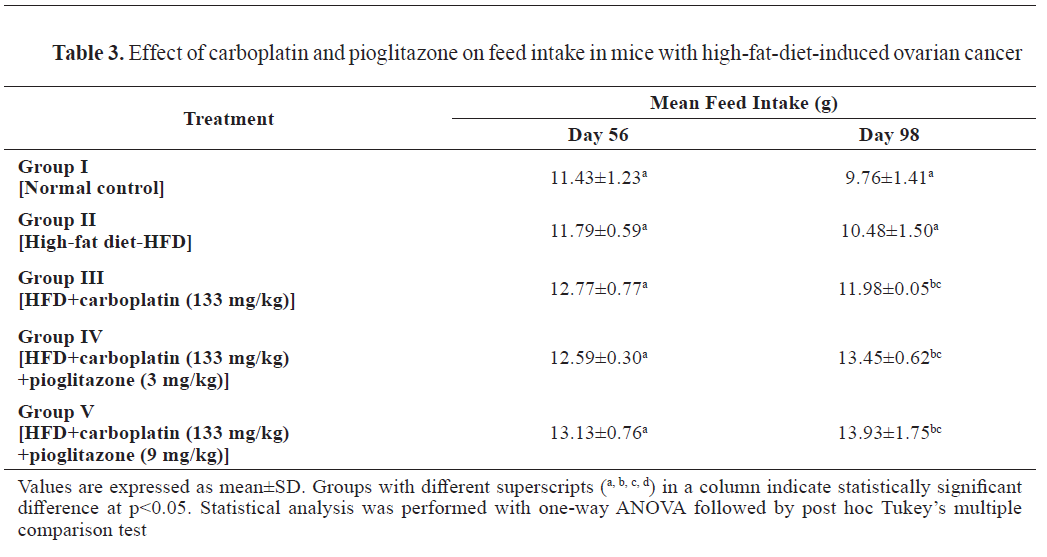
There was no significant mean feed intake difference observed on day 56. On day 98, feed intake significantly (p<0.001) increased in the treatment groups compared to the control and group II (
Table 3).
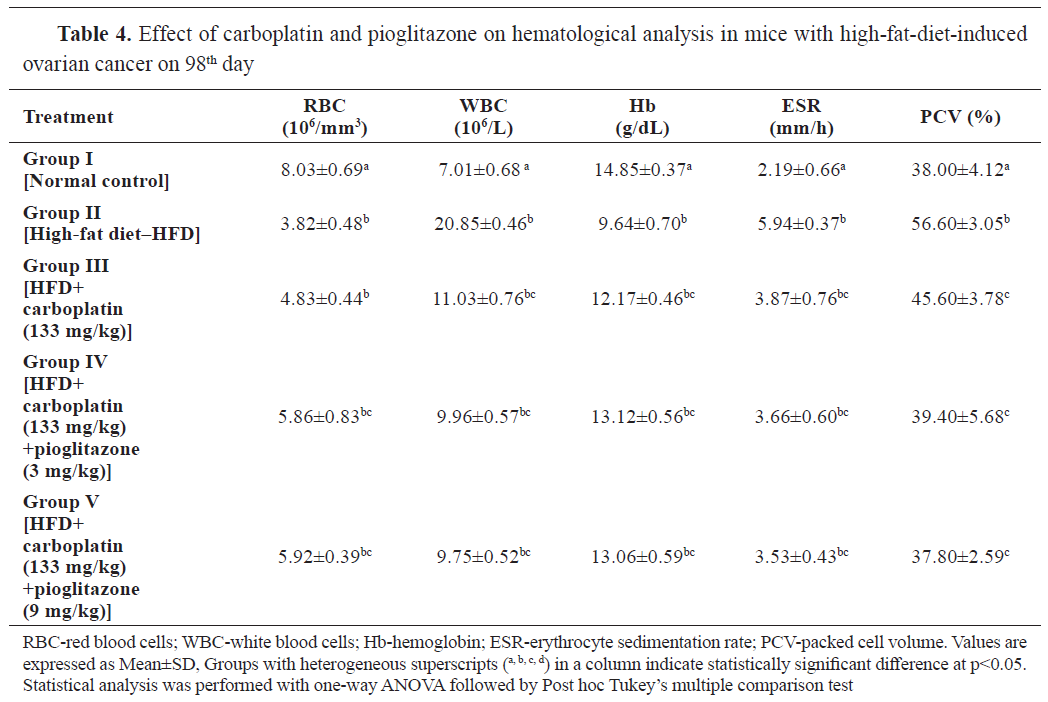
The results in
Table 4 showed the effect of carboplatin and pioglitazone on the hematological parameters in mice with HFD-induced ovarian cancer. The RBC and Hb content were significantly (p<0.001) lower in groups II, III, IV, and V compared to the control at 98 days. The count of WBC was significantly higher (p<0.001) in group II compared to the control. All the treatment groups had significantly lower WBC than group II.
ESR and PCV levels were significantly higher (p<0.001) in group II compared to group I. The treatment groups had significantly lower levels (p<0.001) compared to group I and II. These significant effects were more prominent in the carboplatin and pioglitazone (9 ml/kg) treatment group (group V).
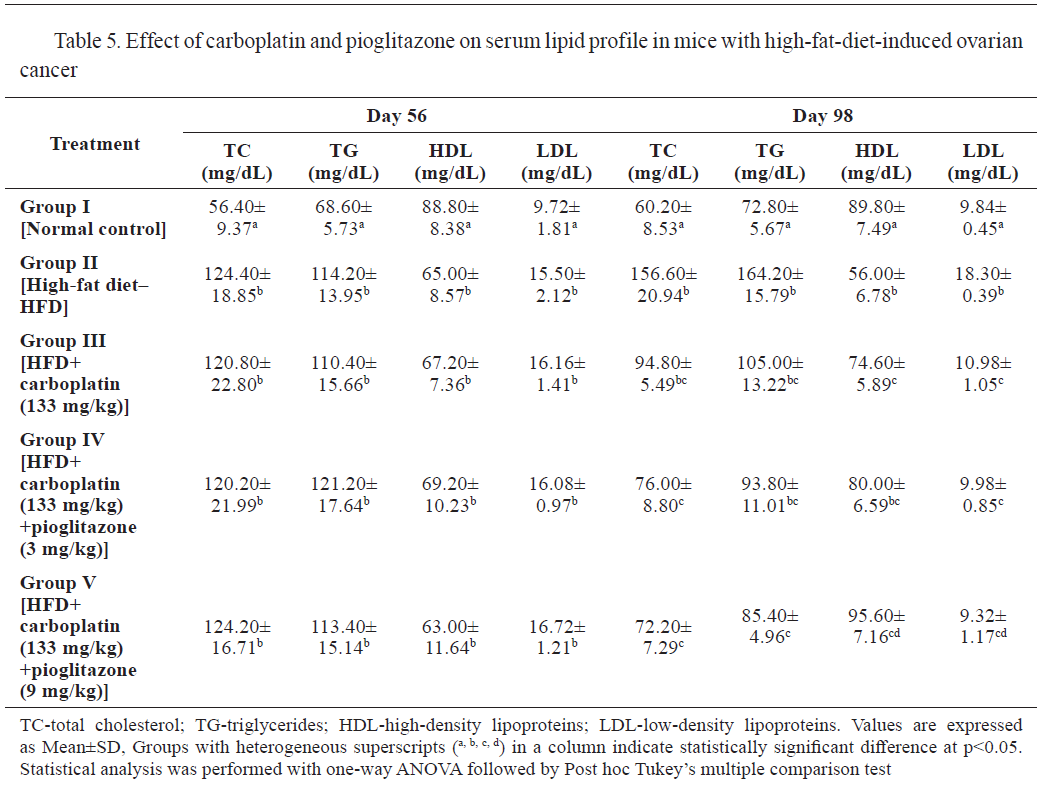
The results in
Tables 5 and
6 show the effect of carboplatin and pioglitazone on the lipid profile and adiponectin levels in mice with HFD-induced ovarian cancer. Groups II to V had significantly higher levels of total cholesterol, triglycerides, and LDL, and significantly lower (p<0.001) levels of high-density lipoprotein (HDL) and adiponectin on day 56, compared to the control.
At the end of the treatment (day 98), TC, TG, and LDL, were significantly higher (p<0.001) and HDL was significantly lower (p<0.001) in groups II compared to the control. The treatments with carboplatin (group III) and the combination of carboplatin with pioglitazone (groups IV and V) yielded significantly lower levels of TC, TG, LDL and significantly increased HDL level compared to group II.
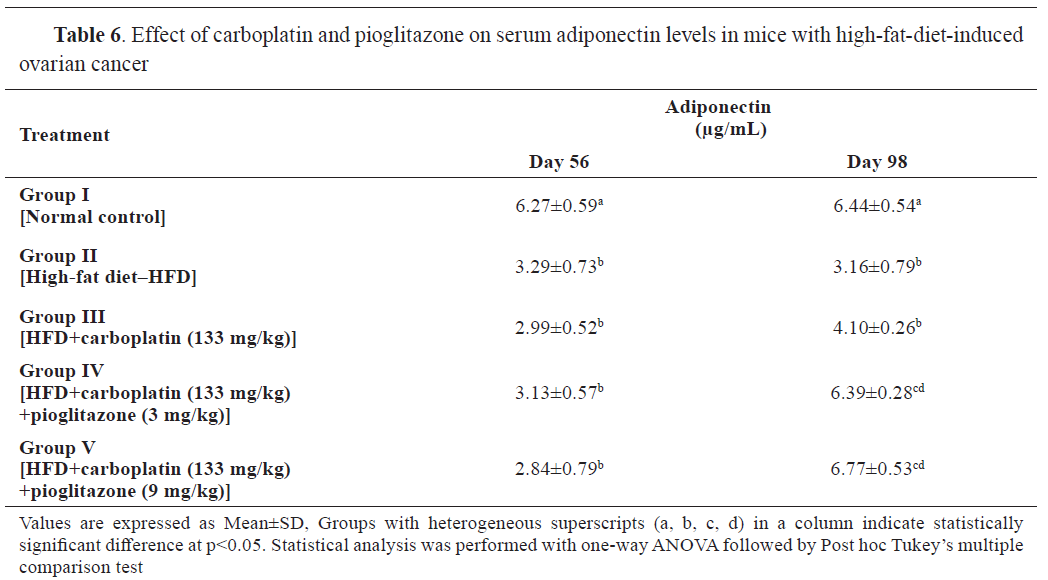
Adiponectin levels at 56 days were significantly lower in groups II-V compared to the control. At 98 days, groups II-IV had significantly lower adiponectin levels compared to the control, whereas group V had significantly higher. Groups II and III had significantly lower adiponectin levels compared to groups IV and V.
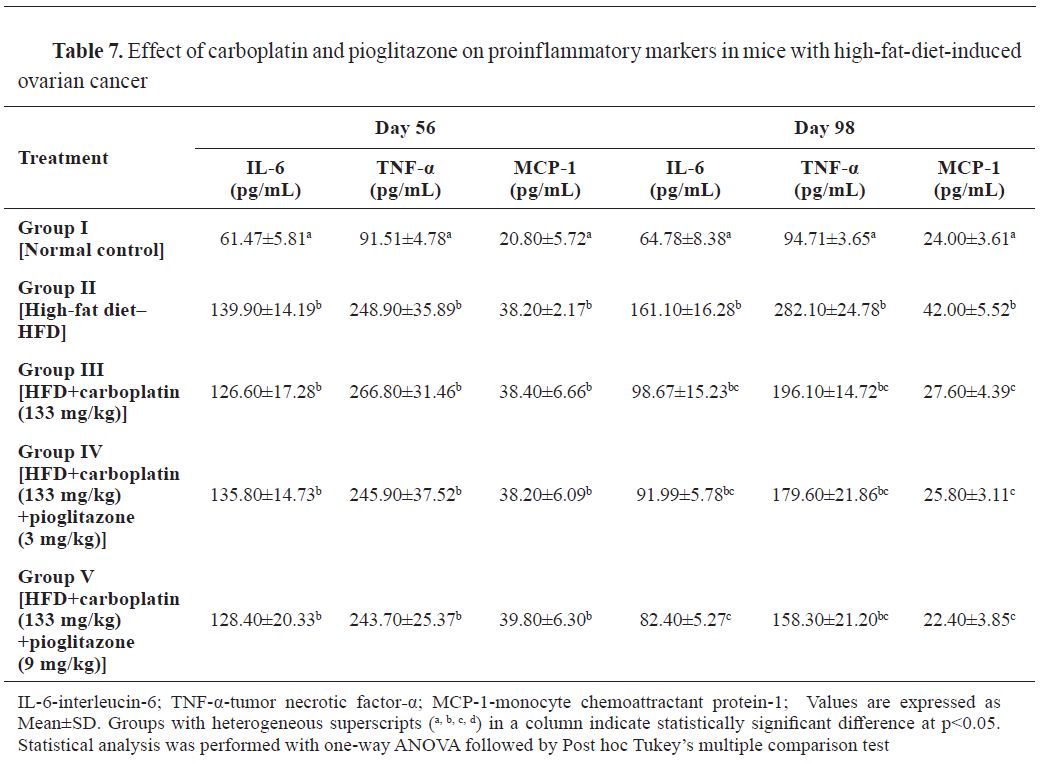
IL-6, TNF-α, and MCP-1 levels were significantly higher (p<0.001) at 56 days in the HFD-fed groups compared to the control. At 98 days, significantly higher (p<0.001) levels of proinflammatory markers were observed in group II compared to the control. IL-6 and MCP-1 levels were significantly (p<0.001) lowered in group V, and also MCP-1 level was significantly (p<0.001) lowered in group VI compared to group II (
Table 7).

At 98 days, groups II, III, IV, and V had 50, 67, 83, and 100% survival rate, respectively. The control had 100% survival rate (
Table 8).
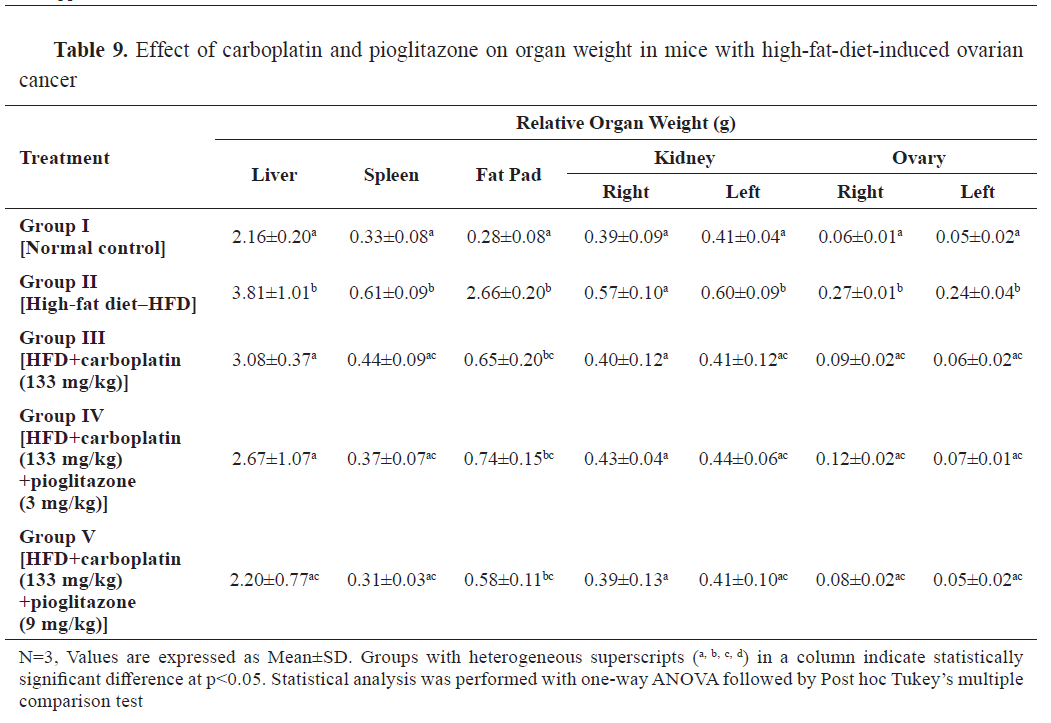
The relative organ weights of liver, spleen, kidney, ovary, and abdominal fat pad were significantly higher in group II compared to the control. The drugs-treated groups effectively reduced the elevated relative organ weights compared to group II (
Table 9).
All treatment groups demonstrated a significantly lower (p<0.001) tumor cell volume than group II. Notably, the groups treated with the combination of carboplatin and pioglitazone (groups IV and V) displayed a significantly lower tumor volume (p<0.001) in comparison to the group III, as shown in
Table 10.
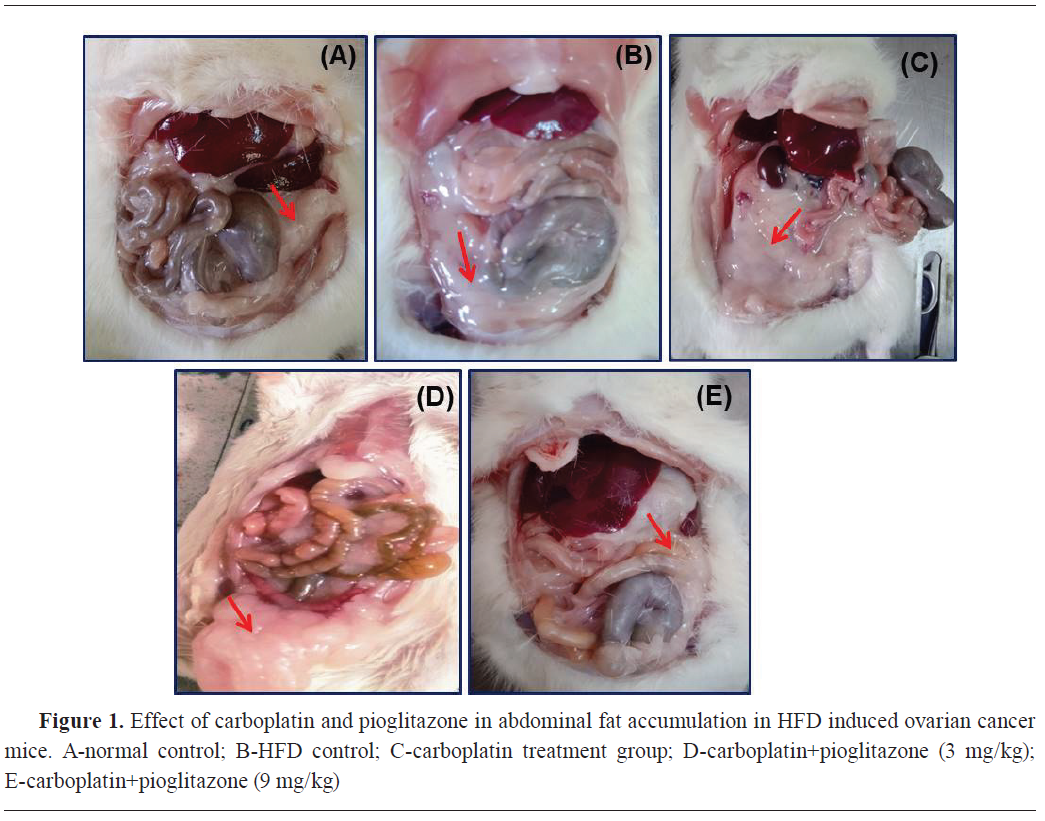
Group II had high fat accumulation in the abdomen. Groups III and IV showed marginally high-fat accumulation in the abdomen. Group V showed lower fat accumulation, similarly as in the control (
Fig. 1).
Group II had high fat accumulation in the reproductive tract and higher numbers of tumorous nodes were observed. Groups III and IV had marginally high fat accumulation in the reproductive tract and fewer tumorous nodes in the ovary. Group V had similar reproductive tract morphology as the control (
Fig. 2).
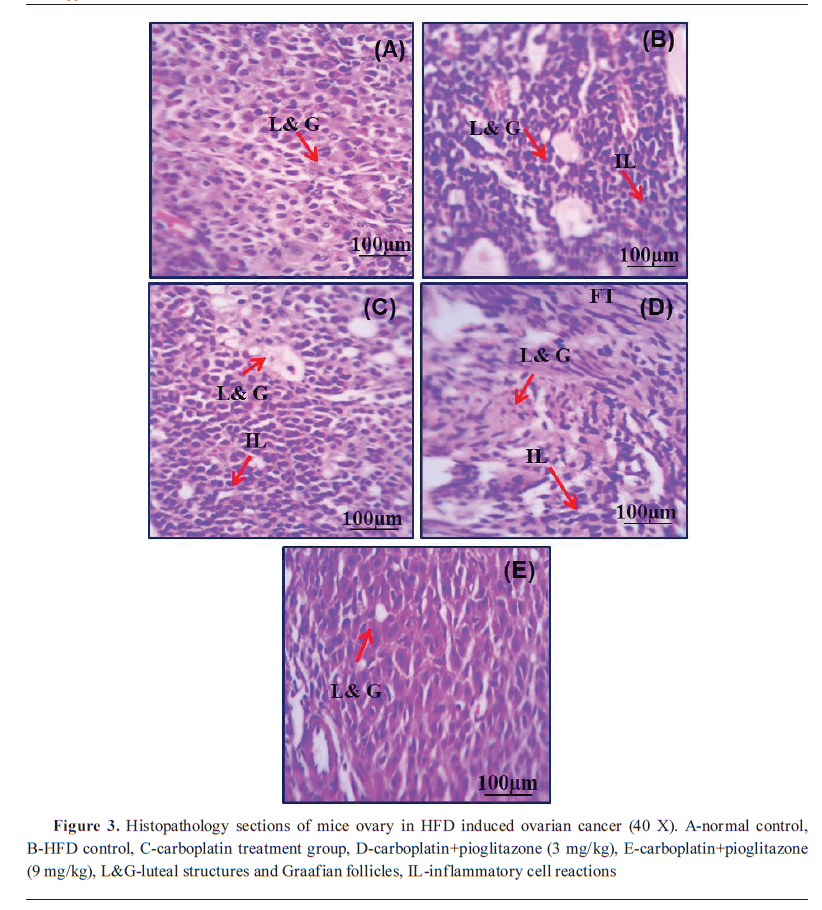
The control had normal ovarian cell structures. Group II had luteal structures and Graafian follicles. Group III had luteal structures and intense interstitial inflammatory reactions. Group III had follicles in different developmental stages with scattered inflammatory cell infiltration. Group V had normal Graafian follicles with mild inflammatory cell infiltration (
Fig. 3).

DISCUSSION
Obesity is linked to the atypical production of hormonal factors, adipokines, cytokines, and inflammatory substances associated with adipose tissue, which are key contributors to ovarian cancer development (
27). In the course of this study, obesity was induced in mice that were given a diet rich in fats. The increased body weight in the HFD disease control established the development of obesity. The combination therapy of carboplatin and pioglitazone significantly reversed HFD- induced obesity by reducing the fat deposition.
Obesity is traditionally linked with a drop in plasma adiponectin levels in humans and animals and a lower expression in adipose tissue. Diminished adiponectin levels might be implicated in the progression of atypical ovarian cancer due to the extended activation of the PI3K/Akt/mTOR signaling system (
28). In this study, the decreased adiponectin level in the disease control group indicates ovarian cancer development in HFD- fed mice. The combination therapy of carboplatin with pioglitazone enhances the level of adiponectin in HFD-induced ovarian cancer, possibly by the activation of the PPAR-γ receptor.
An earlier study found that the diminished concentration of RBC in ovarian cancer induced by a HFD results in the secretion of chemokines from RBC-DARC (Duffy antigen receptor for chemokines). Furthermore, RBCs from HFD-fed mice displayed higher concentrations of reactive oxygen species, membrane phosphatidylserine (PS) externalization, and accelerated phagocytosis by macrophages (
29). This study also shows that the decreased RBC level in the HFD-fed disease control mediates the release of proinflammatory markers. The combination of carboplatin and pioglitazone showed a dose-dependent increase in the level of RBC by inactivating the macrophage binding to the vascular endothelium.
Previous research has demonstrated that HFD-induced obesity and metabolic changes can potentially influence the immune system, including alterations in WBC counts (
30). In this study, the WBC level was considerably elevated in the disease control and confirmed induction of ovarian cancer by the release of inflammatory markers, and also by affecting the immune system. The carboplatin alone and the combination therapy with pioglitazone significantly decrease the level of WBC by altering metabolic changes.
The presence of inflammation and insulin resistance associated with obesity, commonly induced by an HFD, may influence erythropoiesis. Research has shown that obesity-related factors can influence Hb levels and PCV in different ways. Some studies have reported lower Hb levels and PCV in individuals with obesity (
29,
31). The findings of this study reveal that the diminished levels of Hb and PCV in the disease control group validate the occurrence of cancer in mice that were fed a high-fat diet. The combination of carboplatin and pioglitazone significantly increased the level of Hb and PCV in HED-fed mice.
The growth and progression of ovarian cancer are largely contingent upon the inflammatory risk factor. Cytokines that promote inflammation, such as IL-6, TNF-α, and MCP-1, are pivotal in the pathway linking inflammation to cancer progression (
32). The findings of this study also revealed an elevated concentration of TNF-α, IL-6, and MCP-1 proteins in the disease control group of mice. In the context of carcinogenesis, TNF-α exhibits a dual function. On one hand, increased concentrations of TNF-α can harm the tumor vasculature and cause necrosis; on the other hand, it can stimulate the growth of fibroblasts and particular tumor cells. Another major proinflammatory mediator that contributes the carcinogenesis is IL-6 (
33,
34).
IL-6 is acknowledged as a significant mediator of inflammation, influencing the expression of genes that are critical for the advancement of the cell cycle and the suppression of apoptosis, mainly through the activation of the JAK-STAT signaling pathway. The elevated levels of IL-6 have been linked to the development of many malignancies (
35,
36). Some research has revealed that MCP-1 expression is controlled by the MAP kinase and the JAK/STAT pathways. MCP-1 is instrumental in promoting the progression of ovarian cancer. Our findings are consistent with prior research, indicating that elevated levels of proinflammatory cytokines such as IL-6, TNF-α, and MCP-1 in the disease control group, through the inhibition of apoptosis via the JAK-STAT signaling pathway, support the progression of cancer in mice subjected to a high- fat diet. The combination therapy of carboplatin and pioglitazone significantly lowers the level of proinflammatory cytokines by modulating the cell progression through altering the responsible genes expression to the carboplatin alone treatment. It authenticates the anti-cancer potential of carboplatin and pioglitazone in obesity-induced ovarian cancer.
The variance of organ weights was assessed in HFD-induced ovarian cancer mice and regular diet mice. Long-term HFD consumption can cause an elevation in liver weight due to the buildup of fat in the liver cells. The spleen weight increase may be associated with inflammation, immune system activation, or modifications in the localization of immune cells in the spleen. The liver and spleen weight was considerably increased in the disease group showing fat accumulation in the liver and activating the immune system- medicated inflammatory pathway in the spleen. The combination of carboplatin and pioglitazone significantly decreased the liver and spleen weight by reducing liver fat accumulation and suppressing the activation of immune system-mediated inflammatory pathway.
The high-fat diet increases the kidney weight by altering renal pathology like increased collagen accumulation in glomeruli, fat accumulation in kidney, renal medulla infiltration, and sodium homeostatic alteration (
37). The kidney weight was considerably increased in the disease group by inactivating macrophage infiltration and the combination of carboplatin and pioglitazone therapy showed a decrease in the weight of kidneys which is nearer to the normal control group.
The fat pad typically refers to the adipose tissue accumulation in a specific area of the body. The consumption of dietary fats influences the quantity of body fat and the variations in body weight associated with fat accumulation. A high-fat diet is particularly high in saturated and trans-fat, which can accelerate fat accumulation (
38). This study’s results showed that lipid abnormalities like increased LDL, TG, and total cholesterol levels, and decreased HDL and abdominal fat accumulation in the disease group. The combination therapy of carboplatin and pioglitazone decreases the weight of the fat pad and normalizes lipid abnormalities. It indicates that the combination of carboplatin and pioglitazone predominantly reduces the HFD-mediated lipid abnormalities induced by proinflammatory mediator-mediated cancerous conditions.
The previous studies reported that high-fat diet indirectly affects ovarian weight and functions due to obesity and metabolic disturbance. The ovary weight was increased in the HFD- fed disease control due to the alterations in hormone levels, such as adiponectin and leptin. These hormonal changes affect ovarian function and potentially influence weight (
39). The weight of the ovary was increased by elevating the adiponectin and lowering of leptin plasma levels in the disease control. The combination therapy of carboplatin and pioglitazone group decreases the weight of the ovary, which is similar to the normal control; it correlates with the anticancer property of this combination in high-fat diet-induced ovarian cancer conditions.
The quantity of tumor cells present in ovarian cancer can differ significantly based on several factors, including the cancer’s stage and the specific type of ovarian cancer involved. High- fat diets, particularly those abundant in saturated fats, have been linked to obesity-related insulin resistance, which is driven by chronic low- grade inflammation. This condition may play a significant role in the onset and advancement of several cancer types. In individuals with obesity, the surplus of adipose tissue can generate hormones and adipokines that facilitate tumor growth, angiogenesis, and inflammatory processes. These factors may indirectly influence tumors and the progression of ovarian cancer (
40). In this study, the tumor cell volume was increased in the disease control group induced by inflammation. The tumor cell volume was gradually decreased in the combination treatment which might be due to inhibition of the inflammatory response. This finding was also confirmed by the normal ovarian histological structure in the combination of carboplatin and pioglitazone treatment. The combination of carboplatin and pioglitazone (9 mg/kg) showed a 100% survival rate, which also confirms the treatment efficacy of this combination in ovarian cancer.
CONCLUSION
Ovarian cancer in mice was induced by providing fat-rich diet for 98 days. The induction of ovarian cancer in mice was revealed by the increased body weight, relative organ weights, and abdominal fat pad, alteration of serum lipid profile, decreased adiponectin level, increased levels of TNF-α, IL-6, and MCP-1 proinflammatory cytokines, altered histology of the ovary, increased ovarian tumor volume, and reduced survival rate. The combination of carboplatin with pioglitazone, particularly carboplatin (133 mg/kg) and pioglitazone (9 mg/kg), appears to have significant protective effects on ovarian cancer by suppressing the production of proinflammatory cytokines, altering the lipid profile, and enhancing adiponectin production. The proposed mechanism of this beneficial combination is by activating the PPAR-γ receptor to enhance adiponectin production and inactivate the PI3K/mTOR/AKT signaling system, leading to the reduction of proinflammatory cytokines in cancerous conditions. Additional clinical data are crucial for investigating the potential of this combination to enhance carboplatin treatment in cases of obesity-related ovarian cancer.
CONFLICT OF INTEREST
The authors declare that they have no financial or non-financial conflict of interest regarding authorship and publication of this article.
ACKNOWLEDGMENTS
The authors extend their heartfelt appreciation to the Principal of Swamy Vivekanandha College of Pharmacy, along with the Chairman and Secretary of Vivekanandha Education Institutions in Tiruchengode, Namakkal, Tamil Nadu, India, for their significant support and the research facilities they have made available.
AUTHORS’ CONTRIBUTION
SP and SPS designed and carried out the experiments. SPS took the lead in writing the introduction and materials and methods of the manuscript. SP took the lead in results analysis and interpretation of the data. SP took the lead in animal experiments. PT and PP were involved in the analysis of the manuscript. SC and GGV took the lead in writing the discussion part of the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.

 10.2478/macvetrev-2025-0031
10.2478/macvetrev-2025-0031












