INTRODUCTION
Ostriches Struthio camelus var. Domesticus, the only member of the Struthionidae, are the largest flightless birds from the Ratitae family deriving from the Middle Eocene (56 to 33.9 million years ago). As ostriches have a very good ability for acclimatization, they can be farmed all over the world. Currently, ostrich farming due to the rapidly increasing demand for low-cholesterol meat products, leather and eyes used in corneal research and human corneal transplants is undergoing a period of rapid development with the need for scientific data to facilitate understanding of the problematic issues of rearing ostrich chicken and enhancing commercial efficiency of ostrich production (1, 2). As there are notes in literature (3) about the high mortality of ostrich chicken in farms especially until 28 days after hatching (mortality rate of about 46 %), but there is relatively few data about scientific research on organ systems, including the gastrointestinal system at early periods of ontogenesis of ostrich chicken, more detailed scientific research is necessary to be carried out in this field.
In the African ostrich (Struthio camelus var. Domesticus) the stomach is composed of two anatomically and functionally different parts – proventriculus (pars glandularis), where gastric juice secretion takes place and gizzard s. ventriculus (pars muscularis) which is used primarily to grind and break-up food (4). In the small intestine carbohydrates are degraded into monosaccharides, which are the major source of fuel for the metabolism (5).
Although the histological and histochemical features of the gastrointestinal tract of fowl has been studied in great detail already in the last century, whilethe anatomical and histological structure of the African ostrich stomach has been recently studied and described rather intensively (6-10), there are comparatively few studies on the glucose transporters in the gastrointestinal tract of this bird species (11). The aim of the present study was to detect the localization of glucose transporters -2 and -5 (GLUT-2 and GLUT-5) in different parts of ostriches gastrointestinal tract, comparatively in ostriches chicken immediately after hatching in 7 days old and in 30 days old ostriches.
MATERIAL AND METHODS
Material from three parts of the gastrointestinal tract – the superficial gland zone of the proventriculus, duodenum and the terminal zone of the ileum - was collected from 8 female ostriches, raised in the African ostrich farm in Latvia, divided into 3 groups: two ostriches immediately after hatching, three 7 days old ostriches and three 30 days old ostriches. 7 and 30 days old ostrich chicken were housed in a hatching chamber for the first 3 days after hatching, thereafter kept in a box with heated floor. The commercial ostrich chicken’s feed – Strus Premium-Strus 1 and water were available ad libitum. To minimize pain before euthanasia, anaesthesia by 0.5 ml of 10% ketamine and 0.5 ml of 2% xylazine solution intramuscularly was carried out followed by 0.5 ml of 20% pentobarbital intracardiac injection.
Specimens, 0.5-1.0 cm in diameter, were fixed in 10% neutral buffered formalin solution (48h at room temperature), dehydrated in a tissue processor (TISSUE-TEK II) and embedded into paraffin according to the standardized tissue histological procedure (12). Sections 7 μm in thickness were cut (microtome Microm HM360). Slices floated on Poly-L-Lysine coated slides (O. Kindler GmbH, Freiburg, Germany) were deparaffinized with xylene and rehydrated in a graded series of ethanol. The sections were stained using Immunohistochemistry kit (Abcam, UK) according to the manufacture’s guidelines. The sections were pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH 6) for 20 min and incubated with primary rabbit polyclonal antibodies to glucose transporter 2 (GLUT-2) and – 5 (GLUT-5) primary antibodies (Abcam, UK) in 1/1000 dilution, for 30 min at 37° C. Biotinylated secondary antibody and streptavidin-conjugated peroxidase were used for detection using DAB as chromogen. Nuclei were counterstained with Harris Hematoxylin. Negative controls contained antibody diluent (Dako, S0809) instead of primary antibodies. Rat liver tissue sections for identifying GLUT-2 and human kidney tissue sections for GLUT-5 were used as positive controls available for comparison on Abcam antibody producer’s homepage (http://www.abcam.com) as examples for the antibodies immunohistochemistry on paraffin-embedded tissues (IHC-P).
Photos of the slides were taken by the microscope Zeiss Axioplan-2 Imaging (Germany) and saved to the computer for analyzing by visual control using camera (AxioCam HRc, Germany) connected to the microscope.
The experiments were carried out in accordance with the Guidelines laid down by the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the Ethical Committee of Latvian University of Agriculture has approved the experiments (protocol number 2014/2).
RESULTS
The investigation provided information about the localization of GLUT-2 and GLUT-5 in gastrointestinal tract in ostrich chicken in different ages in their early ontogenesis (Table 1).
Table 1. The expression of GLUT-2 and GLUT-5 in the gastrointestinal tract of ostriches in different ages
In ostrich chicken after hatching, the staining for glucose transporters GLUT-2 and GLUT-5 occured to be very weak in all investigated regions of the gastrointestinal system, positively were stained only the cytoplasm of some epithelial cells of proventriculus and the cytoplasm of the enterocytes (absorptive cells) in the apical parts of intestinal villi. The brush border membranes of the villi were stained weakly and the goblet cells in the epithelium of the small intestine were mostly unstained (Fig. 1).
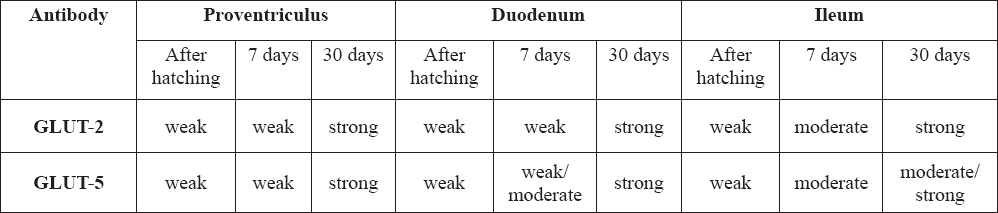
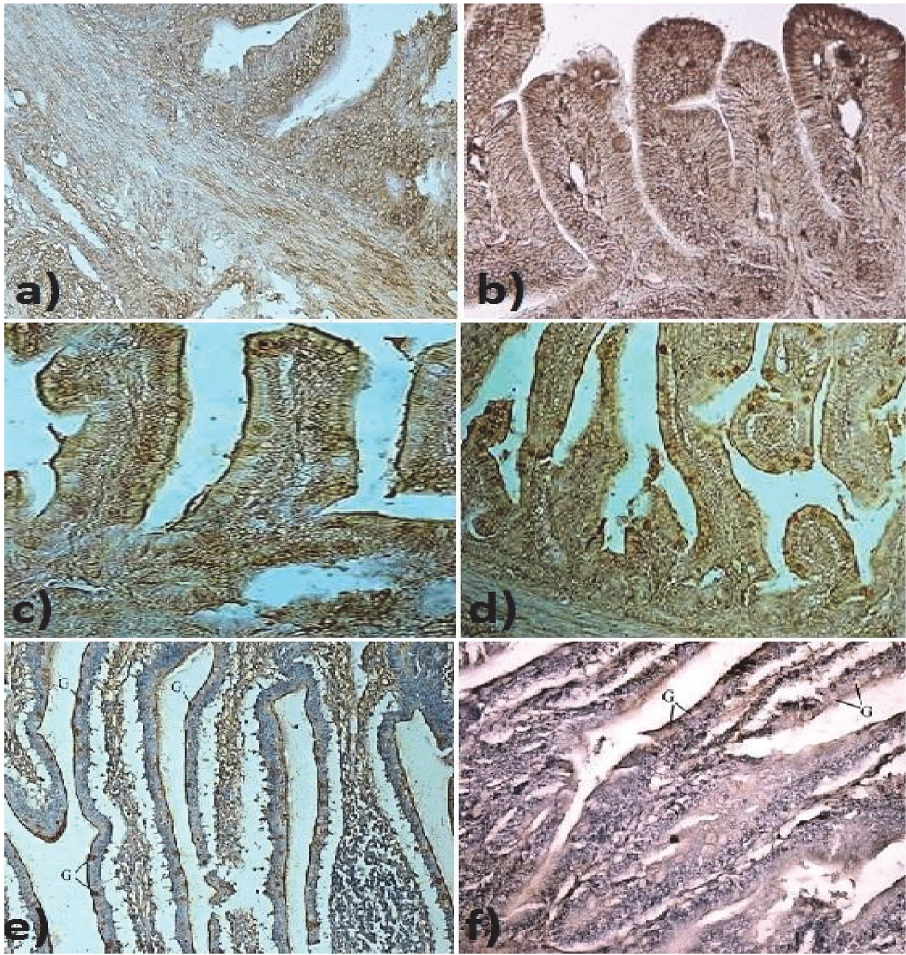
Figure 1. Glandular stomach: a) Epithelial cells weakly stained for GLUT-2 in proventriculus of ostriches after hatching. 400x; b) Epithelial cells weakly stained for GLUT-5 in proventriculus of ostriches after hatching. 400x; c) Weak staining for GLUT-2 in 7 days old ostriches proventriculus. 200x; d) Staining for GLUT-5 in 7 days old ostriches proventriculus. 200x; e) Strong staining for GLUT-2 in 30 days old ostriches proventricular epithelial cells (arrows). 200x. f) Strong staining for GLUT-5 in 30 days old ostriches proventricular epithelial cells (arrows). 200x.
In 7 days old ostriches, similarily to the chicken after hatching, the staining for both antibodies occured to be weak in the epithelial cells of proventriculus and moderate in duodenum, however comparatively the staining was stronger for GLUT-5 in duodenal epithelial cells. Moderate staining for both antibodies was noted in the goblet cells of the terminal zone of ileum (Fig. 2).
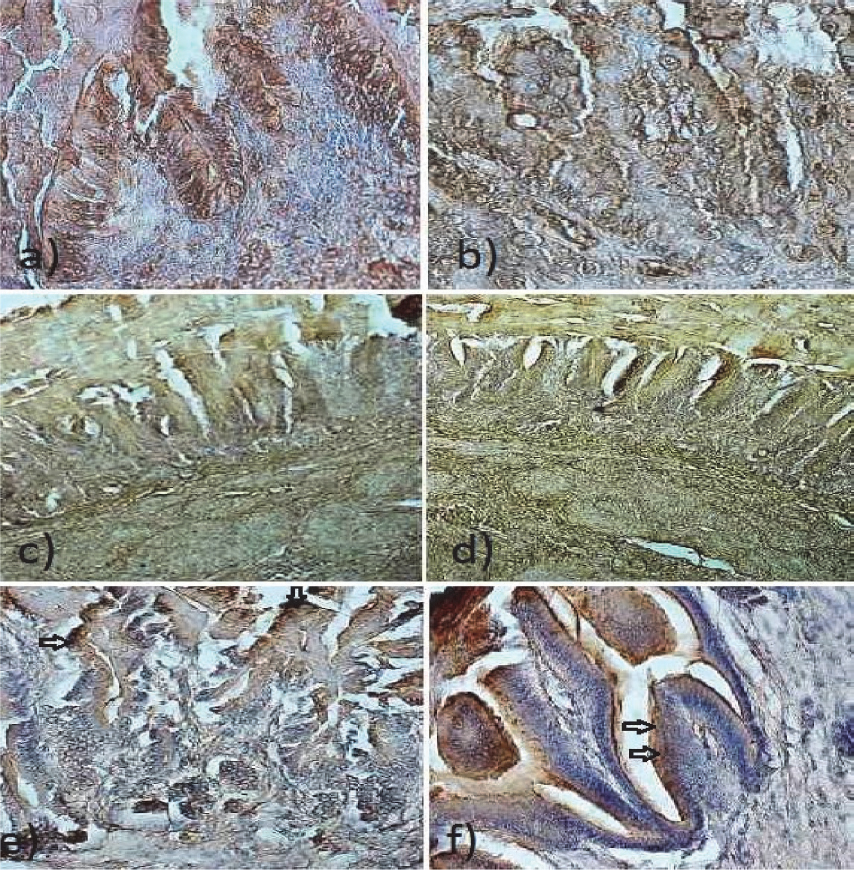
Figure 2. Duodenum: a) Weak staining for GLUT-2 in epithelium of duodenal mucosa of ostriches after hatching. Note the unstained goblet cells in the epithelium. 200x; b) Weak staining for GLUT-5 in epithelium of duodenal mucosa of ostriches after hatching. Goblet cells in the epithelium remain unstained. 200x; c) Weak staining for GLUT-2 in epithelial cells of the duodenal villi of ostriches after hatching. 200x; d) Moderate staining for GLUT-5 in epithelial cells of the duodenal villi of ostriches after hatching. 200x; e) Brush border membranes and goblet cells (arrows) strongly stained for GLUT-2 in epithelium of duodenal mucosa of 30 day old ostriches. 200x.; f) Brush border membranes and goblet cells (G) strongly stained for GLUT-5 in epithelium of duodenal mucosa of 30 day old ostriches. 200x.
In 30 days old ostriches epithelial cells of proventriculus, the brush border of enterocytes as well as the goblet cells in the small intestine were stained strongly positively for GLUT-2 and GLUT-5. In the ileum, the staining was more intensive for GLUT-2 (Fig. 3).
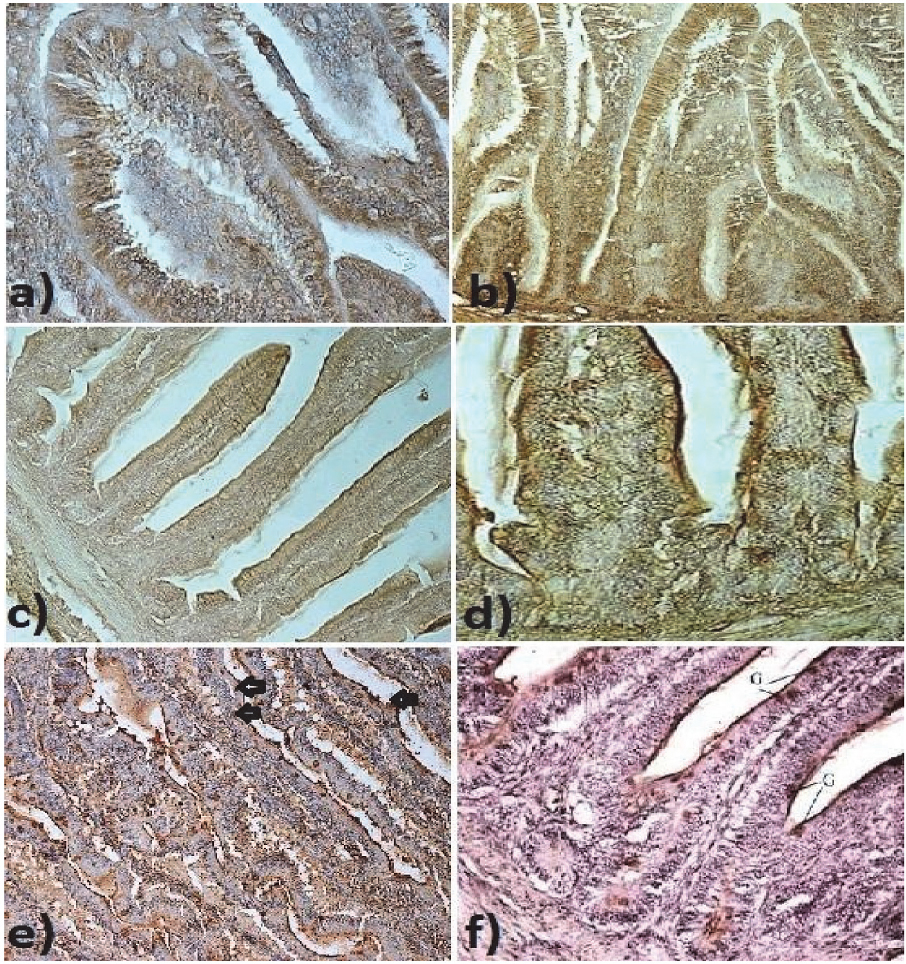
Figure 3. Terminal zone of ileum: a) Weak staining for GLUT-2 in ileal epithelium in ostriches after hatching. 400x; b) Weak staining for GLUT-5 in ileal epithelium in ostriches after hatching. 400x; c)Brush border membranes and goblet cells moderately stained for GLUT-2 in duodenal epithelium in 7 days old ostriches. 200x; d) Brush border membranes and goblet cells strongly stained for GLUT-5 in duodenal epithelium in 7 days old ostriches. 200x; e) Strong positive staining for GLUT-2 in goblet cells (G) in the terminal zone of ileum of 30 day old ostriches. 100x.; f) Positive staining for GLUT-5 in goblet cells (G) in the terminal zone of ileum of 30 day old ostriches. 100x.
DISCUSSION
According to the farmers observations there is a well-established opinion that it is advisable to start the feeding of ostrich chickens not earlier than on the third or fourth day after hatching, because starting feeding earlier increases mortality of ostrich chickens (13). However this opinion lacks scientifically proven arguments. The researcher’s opinions on the appropriate starting time of feeding differ (14). As the optimal time of the first feeding of ostrich chickens mainly depends on how well developed and functioning is the digestive tract at the moment of hatching. More studies of the gastrointestinal tract are needed at the early stages of ontogenesis.
As glucose transporters play a pivotal role in the transfer of glucose across epithelial cell layers that separate distinct compartments in the organism (15, 16), in our experiments on ostrich chickens the localization of GLUT-2 and -5 was observed in three different parts of the gastrointestinal tract. GLUT-5 is the fascilitated fructose transporter which allows for fructose to be transported from the intestinal lumen into the enterocyte by facilitated diffusion due to fructose’s high concentration in the intestinal lumen and is shown to be located in the mammals’ intestinal epithelium (17). After apical transport mediated by GLUT-5, fructose is transported across the basolateral membrane by GLUT-2. GLUT-2, a transmembrane carrier protein that enables passive movement of hexoses across cell membranes, was used since the hexoses’ transport in birds occurs predominately by passive transport (18, 19). Recent works (20) propose that GLUT-2 is also involved in the apical transport of hexoses (glucose, galactose and fructose).
We showed that in all investigated regions of ostrich chickens shortly after hatching, compared to 7- and 30 days old chicken, the staining for both glucose transporters was weak - the brush border membranes of the intestinal villi and the goblet cells in the epithelium of small intestine were mostly unstained. In 7 days old ostriches, the glandular stomach and duodenum stained weak, however the epithelial cells of the terminal zone of the ileum were stained intensively for GLUT-5 (fructose transport).
In 30 days old ostriches, the epithelial cells of glandular stomach as well as brush border membranes and goblet cells in the duodenal and ileal epithelium were stained intensively positively for GLUT-2 and GLUT-5. The staining for GLUT-2 occurred to be stronger in the terminal part of the ileum, indicating a stronger transport of hexoses in this region. These experimental results may indicate that the gastrointestinal tract of ostriches immediately after hatching is not yet entirely capable of transportation of carbohydrates, hence the ostrich chickens mostly begin to eat only at the end of the first week after hatching.
CONCLUSION
The investigation provided comparative information about the localization of GLUT-2 and GLUT-5 in gastrointestinal tract in ostrich chickens at different ages in their early ontogenesis.
In our study in ostrich chickens immediately after hatching, the staining for glucose transporters GLUT-2 and GLUT-5 occurred to be very weak. In 7 days ostriches, the staining for the glucose transporters was stronger especially in the epithelium of the terminal zone of the ileum. In 30 days old ostriches epithelial cells of proventriculus, the brush border of enterocytes as well as the goblet cells in the small intestine were all stained strongly positively for GLUT-2 and GLUT-5.
The results of our study may indicate the possibility of close relationship between feeding and ability to transport sugars in the gastrointestinal tract. The knowledge about the transepithelial transport of sugars in the gastrointestinal tract of ostrich chickens in their early ontogenesis gives valuable information about their feeding requirements.