INTRODUCTION
Bluetongue (Febris catarrhalis ovium) is a non-contiguous vector-borne viral disease of domestic and wild ruminants with a wide distribution and considerable impact on the health status and production (1, 2). The disease is caused by the Bluetongue Virus (BTV), a double stranded RNA virus of the Orbivirus genus in the Reoviridae family (3, 4). There are currently 26 different BTV serotypes (5). The most important route of BTV transmission is primarily through the bite of haematophagous midges of the genus Culicoides (Diptera: Ceratopogonidae), the major biological vector of the virus (6, 7). In endemic regions the disease displays a seasonal pattern, with highest incidence in late summer when the vector population reaches its peak (8). The severity of clinical symptoms depends mainly on the infected host, the virulence of the BTV strain and the immunological status of the affected animals (2, 3). The clinical form of bluetongue is most commonly observed in domestic sheep, and it is characterised by fever, excessive salivation, hyperaemia of the nasal and labial mucous membranes, oedema and swelling of the face, severe dyspnoea, asphyxia, oedemas and a blue swollen tongue partly hanging out of the mouth (9, 10, 11). In cattle, goats and wild ruminants, bluetongue is most often present in a subclinical form or with mild or transient clinical signs (2, 12). Occurrence of bluetongue is closely related with the activity of competent Culicoides vectors, thus the emergence of this disease was traditionally reported in a geographic band between the latitudes 40°N and 35°S, which is its natural habitat (14).
Until 1998, only short outbreaks of bluetongue were occasionally recorded in South European countries (10). However, after nearly 20 years of absence BTV was recorded again on four Greek islands, where BTV-9 was isolated and that was the first report of this serotype on the European continent (15, 16). Since 1998, multiple emergences of bluetongue have been evidenced in Southern Europe, caused by various serotypes of the virus: 1, 2, 4, 8, 9, 10 and 16 (14, 17, 18). From 2006-2010 an epidemic of bluetongue caused by BTV serotype 8 was recorded in many countries of Central and Northern Europe (14, 17, 19). There have been indications that serotypes BTV-6 (detected in Germany and Holland) and BTV-11 (detected in Belgium) could have been introduced by illegal import of live attenuated vaccines (20, 21), as no virus has been isolated and no clinical signs of bluetongue disease have been observed (22).
The first report of bluetongue in Serbia was in 2001 (23), when BTV-9 was detected. In the same year, BTV-9 positive cases were also reported in Bulgaria, Macedonia, Montenegro and Croatia (10, 24, 25), and in the following year, the same serotype was confirmed in Bosnia and Herzegovina (10). In September 2014, following the outbreak of clinical symptoms of bluetongue in sheep and cattle in Serbia, the Institute of Veterinary Medicine Belgrade (Serbia) and the Pirbright Reference Laboratory (England), confirmed the presence of BTV-4 for the first time in this country. Date of the first confirmed case was September 3, 2014 (http://www.oie.int/wahis_2/public/wahid. php/Reviewreport/Review?reportid=15987). The outbreak of BTV in Europe has caused considerable economic losses, including not only direct damage due to mortality and reduced production, but also indirect damage because of trade bans of ruminants between BTV infected and non-infected areas (17, 26). Due to the increasing importance of bluetongue, the fast spreading of the causative agent and the abovementioned direct and indirect losses, this disease has been included in the List A of notifiable diseases compiled by the OIE (27).
This paper presents a retrospective analysis of the incidence and geographical distribution of BTV in sheep, cattle and goats in the 2014 BTV outbreak in Serbia.
MATERIAL AND METHODS
Investigation of BTV positive animals
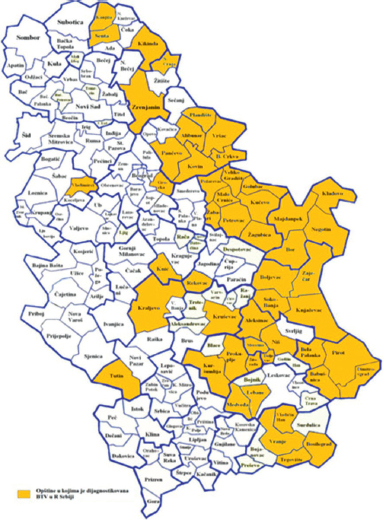
The epizootiological situation on Serbian territory is under the surveillance of 12 veterinary institutes, where each institute covers specific area (Fig. 1). The presence of bluetongue was investigated in sheep, cattle and goats. Detection of clinically infected animals throughout Serbian territory was carried out in a joint action by field veterinarians, specialists from veterinary institutes and representatives of the Veterinary Directorate (Ministry of Agriculture and Environmental Protection of Republic of Serbia). In an extensive action that lasted from 03.09 - 31.12.2014, all cases suspected for bluetongue disease were reported by field veterinarians to the veterinary institute responsible for each district. Blood sample from each suspect animal was subjected to serological and molecular testing according to the recommendation from OIE (28). Subsequently, blood from the rest of animals from herds with confirmed BTV positive cases was also tested in the same manner. Testing for the presence of antibodies against BTV was performed using BTV antibody competitive ELISA (cELISA) (IDVET, Montpellier, France). In each seropositive case detection of BTV RNA was performed using real-time RT-PCR (29). All laboratory analyses were performed by the National Reference Laboratory for bluetongue disease, The Institute of Veterinary Medicine - Belgrade (Serbia).
Figure 1. Distribution map of bluetongue cases in territories covered by veterinary institutes in Serbia in 2014
Statistical analysis
Results are shown in a form of descriptive statistical parameters. Differences were tested using t-test in cases of two independent samples. Data analysis was performed using software package PASW Statistics 18.
RESULTS
In 2014, 644 outbreaks were reported in 49 municipalities in 17 (out of 29) administrative regions in Serbia (source: Veterinary Department, Ministry of Agriculture and Environmental Protection, Republic of Serbia, http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?reportid=15987). Bluetongue cases were confirmed on territories under the surveillance of the following 9 veterinary institutes: Nis, Pozarevac, Zajecar, Pancevo, Zrenjanin, Kraljevo, Sabac, Jagodina and Belgrade (Fig. 1). From the total number of animals with clinical signs of bluetongue, 91.45% were positive on cELISA testing. Results of molecular identification confirmed the presence of BTV virus serotype 4 in 56.78% of seropositive samples. With regards to the total number of susceptible animals (1 484 725) kept in the areas of BTV outbreak recorded in 2014, the overall incidence proportion of BTV in Serbia reaches 0.16% (2 313 infected animals).
Bluetongue in sheep
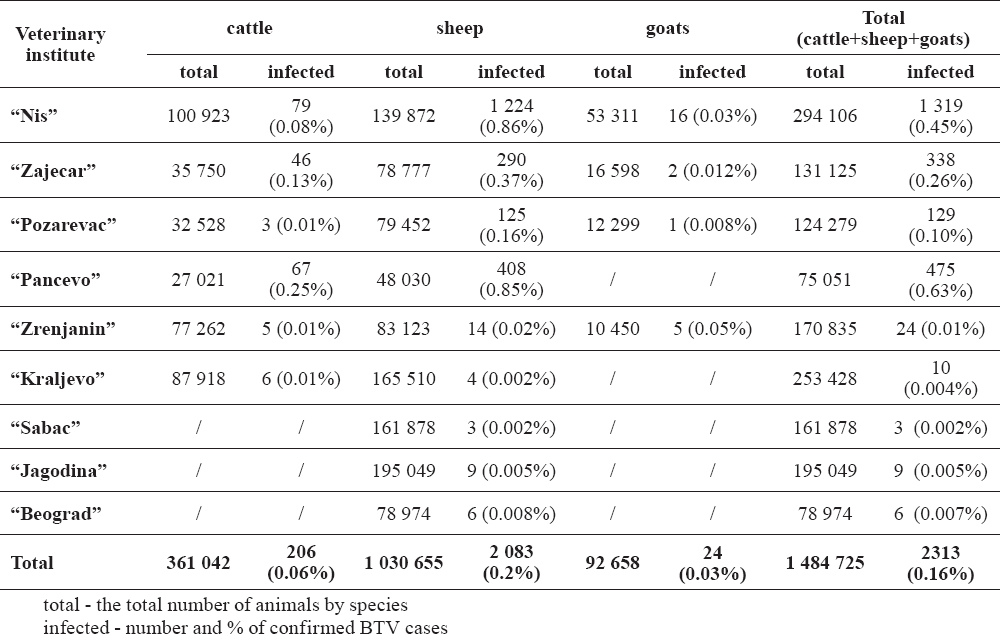
Bluetongue cases in sheep were reported on territories covered by 9 veterinary institutes. From the total number of sheep in Serbia (n=1 748 110), 58.96% (n=1 030 655) were located in areas affected by bluetongue (Table 1). Total of 2 083 cases of infected sheep were reported during the investigation period, which represents 0.2% of the total number of sheep in affected areas. The highest incidence proportion with 1 224 cases per 139 872 sheep or 0.88%, was recorded in the territory under surveillance of Veterinary Institute of Nis (Table 1).
Table 1. Distribution of bluetongue infection in 2014 on territories under the surveillance of veterinary institutes in Serbia
Bluetongue in cattle
In cattle, BTV-4 was confirmed in 6 out of 12 areas covered by veterinary institutes. From the total number of cattle in Serbia (n=920 068), 39.28% (n=361 042) were located in areas affected by bluetongue. A total of 206 infected cases per 361 042 of cattle were reported, which gives incidence proportion of 0.06%. The highest incidence proportion with 79 cases per 100 923 of cattle or 0.08%, was recorded in the territory under surveillance of the Veterinary Institute of Nis.
Bluetongue in goats
Bluetongue in goats was confirmed on territories under the surveillance of 4 veterinary institutes. Total of 24 infected cases per 92 658 goats were reported, which gives incidence proportion of 0.03%. The highest incidence proportion with 16 cases per 53 311 goats or 0.02%, was recorded in the territory under surveillance of the Veterinary Institute of Nis.
Monthly distribution of bluetongue cases
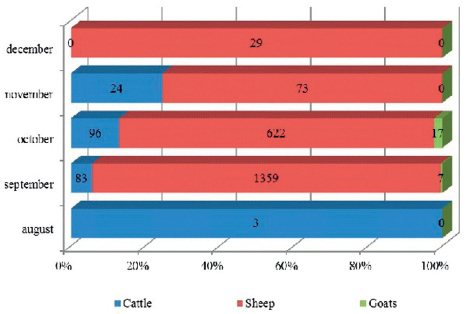
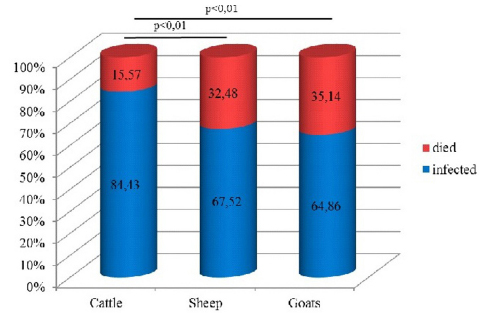
The highest incidence of BTV in all susceptible species was documented during the period September-December 2014, with different incidence rates for each species. Bluetongue cases in cattle and sheep were not recorded only in December and August, respectively. BTV positive goats were reported only in September and October. Results of monthly incidence proportion of bluetongue cases, regardless of the species, showed 94.43% positive cases in September and October, which is significantly higher compared to other months (p<0.01). With regards to the species, the highest incidence of BTV and mortality were detected in sheep. Recorded lethality (%, died/infected x 100) in cattle, sheep and goats was 18.45% (38/206 x 100), 48.10% (1002/2083 x 100) and 54.17% (13/24 x 100), respectively (Fig. 2A, B).
Figure 2A. Morbidity - lethality ratio of bluetongue infected ruminants in Serbia in 2014
Figure 2B. Morbidity - lethality ratio of bluetongue infected ruminants in Serbia in 2014
DISCUSSION
During the past 20 years, the distribution and characteristics of BTV infection have changed substantially in Europe. It has been suggested, but not proven, that global climate change is responsible for spreading the areal of BTV vectors and enabling the vector competence to indigenous Culicoides species, which influenced the occurrence of different BTV serotypes in geographic areas above 50°N (11). The presence and geographic distribution of Culicoides biting midges have proven to be critical aspects in BTV transmission among susceptible animals, and also the limiting factor in occurrence of the disease (3). Prior to 1998, the appearance of BTV infections were limited to a geographic band between the latitudes 40°N and 35°S, habitat of its main vector, C. imicola (14). The breaking point for emergence of bluetongue on the Balkans was the 1998 BTV outbreak on 4 Greek islands (30, 31). At the same time, with the exception of Turkey, this was the first report of BTV-9 in this part of the Europe (32). During the following years, in addition to BTV-9, the presence of BTV-1, BTV-2, BTV-4 and BTV-16 was also confirmed (10, 17).
The first outbreak of bluetongue in Serbia was reported at the end of 2001, where BTV-9 was detected (23). There is a presumption that BTV-9 reached Serbia through infected host animals from Macedonia, Albania and Bulgaria (10), from where it spread further to Croatia (33, 34) and Bosnia and Herzegovina (10). During the outbreak in 2002, bluetongue cases in Serbia were recorded in 37 focal points within 16 municipalities (23). The most recent outbreak of BTV in Serbia was reported at the end of August 2014 (35), and the virus was identified as BTV-4. This was the first report of BTV-4 in Serbia, after which its presence was reported in Montenegro, Bosnia and Herzegovina and Croatia (36). Outbreaks of BTV-9 and BTV-4 in Serbia are most likely connected to the vector competence of other species of Culicoides other than C. imicola (7). Isolation of BTV-9 and BTV-4 from Palaearctic Culicoides species, especially C. obsoletus, C. pulicaris and C. scoticus indigenous to the Balkans (17) supports the hypothesis that other species of biting midges act as primary BTV vectors.
Bluetongue cases in Serbian ruminants were recorded during 644 outbreaks in 49 municipalities, i.e. 17 administrative regions covered by 9 veterinary institutes. With regards to the total number of susceptible animals kept in the areas of BTV outbreak recorded in 2014, the overall incidence of BTV in Serbia reaches 0.16%. Individual incidence for sheep, cattle and goats was 0.2%, 0.06% and 0.03%, respectively. This is consistent with the incidence proportions recorded in Greece, Bulgaria and Macedonia (37). BTV-4 outbreak was reported in Croatia and Bosnia and Herzegovina in 2014, but official statistics concerning number of infected and deceased animals has not been available to the authors of this paper.
The highest number of bluetongue cases was documented in September and October. These results support the observation that the highest incidence of BTV corresponds to the period of greatest vector abundance (38). Investigation of the bluetongue outbreak indicated higher number of infected animals in municipalities positioned in the basins of major rivers that flow through Serbia (Danube, Sava, Velika Morava, Timok). Highest number of fatality cases due to BTV infections in Serbia, in 2014, was recorded in sheep, followed by goats and cattle. This is not surprising given that sheep develop the most severe clinical conditions, while cattle are the most susceptible host (10). Surprisingly high lethality recorded in goats is in disagreement with the traditional views that bluetongue in goats and cattle often remains subclinical or exhibits mild and transient signs (12, 13).
In comparison with the BTV-9 outbreak in Serbia in 2002, BTV-4 outbreak in 2014 resulted in substantial increase in the number of affected areas. Potential reasons for this could be : (i) spread of Culicoides biting midges with regards to their abundance and vector competence due to climate changes (39); (ii) increase in ungoverned and illegal trade of ruminants from BTV affected surrounding areas (Bulgaria, Macedonia and Albania). For the purpose of increasing the understanding of occurrence and distribution of BTV in Serbia, along with investigation of infected animals, a survey aimed at defining the Culicoides species present in Serbia, their vector competence and infection status is urgently needed.
CONCLUSION
From the above stated, monitoring of bluetongue disease in Serbia relies on active surveillance programmes aimed at: (i) identification and tracing of susceptible and potentially infected animals and (ii) detection, distribution and prevalence of insect vectors. In addition, restriction and trade bans of ruminants between BTV infected and non-infected areas are conducted. For 2016, vaccination of sheep against BTV is planned to be implemented in areas affected by bluetongue in 2014 and 2015.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.