INTRODUCTION
Bovine tuberculosis (bTB) is an infectious chronic disease of cattle worldwide. Besides cattle, the causative agents of bTB can affect other domestic animals, a wide variety of wildlife species and humans (1). Although the disease in cattle is mainly caused by Mycobacterium bovis (M. bovis) and to a lesser extent by Mycobacterium caprae (M. caprae) (2), according to the Task Force Bovine Tuberculosis Subgroup (3), bovine tuberculosis is the “Infection in cattle with any of the disease causing mycobacterial species within the Mycobacterium tuberculosis complex”. Despite the differences in host specificity, the members of the Mycobacterium tuberculosis complex (MTBC) have 99.9% similarity at a nucleotide level and an identical 16s rRNA sequence (4).
Bovine tuberculosis is endemic in many European countries and has a major impact on their economy and agricultural industries (5). The disease has a significant public health importance, especially in developing countries where the burden of M. bovis infection in humans is important, having in mind the close habitat of the animals and humans, HIV/AIDS condition, consumption of non-pasteurized milk and milk products and the level of veterinary control (6). According to Muller et al. (7), zoonotic tuberculosis in Europe is represented by a median proportion of 0.4% (0%-21.1%) of M. bovis and M. caprae in all bacteriologically confirmed human cases, whereas for Africa the median proportion is 2.8% (0%-37.7%).
Republic of Macedonia (RM) is a non Officialy Tuberculosis Free (nonOTF) country and despite the eradication program in force, the disease has been present for many decades. According to Gramatikovski and Stojanovski (8), the first written data dates from 1926 when the disease was first diagnosed in imported cattle. According to Nikolovski et al. (9), in the period from 2007 till 2009, bTB was diagnosed (by intradermal comparative cervical tuberculin test) in 0.24% of the tested cattle. The baseline of the eradication program is the test and slaughter policy (10). The testing is performed by an intradermal single cervical test (ISCT), which if positive or suspicious is followed by an intradermal comparative cervical test (ICCT). All cattle that have reacted positive to ICCT are slaughtered, but post mortem inspection and laboratory confirmation are not regularly conducted. So far, the data from the tuberculin skin test are the only diagnostic criteria that certify the presence of bTB in RM, which indicates a lack of scientific evidence about the etiology and epidemiology of the disease. Considering this, the aims of this study were to determine the presence and distribution of typical tuberculous lesions in reactor cattle and to isolate and identify the causative agents of bTB in RM.
MATERIAL AND METHODS
Sample collection
The study was conducted on cattle slaughtered due to a positive reaction on an ICCT as part of the National eradication program in 2011, 2012 and 2013. The samples originated from 188 animals from 101 holdings in 18 epidemiological units (municipalities) in RM. The number and origin of the samples are shown in Table 1.
Table 1. Number and origin of the tested samples
One-hundred-and-forty-five samples (77%) originated from 95 different holdings where the animals were condemned due to one outbreak. Twenty four samples (12,7%) were collected from animals derived from 5 holdings due to two outbreaks. Nineteen samples (10%) originated from animals confiscated for slaughter from one holding (herd with chronic bTB) during the three year research period.
The samples were collected during slaughtering of the animals and consisted of the retropharyngeal (RLNs), mediastinal (MdLNs), and mesenteric (MsLNs) lymph nodes (regardless of the presence or absence of lesions). Also, if pathological changes on the lungs, liver or other organs were present, those organs were sampled as well. The samples were transported (at +4°C) and processed in the laboratories of the Faculty of Veterinary Medicine in Skopje. The samples were longitudinally sliced on 5 mm thick slices and the presence of tuberculous lesions was recorded.
Isolation of mycobacteria
For bacteriological testing, the sampled organs from one animal were pooled and processed by homogenization, decontamination and concentration, and were inoculated on selective media [Lowenstein Jensen (LJ) with glycerol (Biolife, Italy), LJ without glycerol (Oxoid, UK) and Stonebrink medium supplemented with pyruvate (Oxoid, UK)] as previously described (11, 12, 13). The incubation was in aerobic atmosphere, at 37°C for 12 weeks. In case of contamination, the procedure was repeated. Colonies with pale yellow color and dysgonic growth that appeared after min. 4 weeks of incubation were classified as suspect colonies and were further processed by molecular methods.
Molecular identification
The extraction of the mycobacterial DNA from the suspect colonies using PureLinkGenomic DNA Kit (Thermo Fisher Scientific Inc.) was performed according to the producer’s manual. The molecular detection and identification of the mycobacteria was based on the presence of the 16S rRNA, as well as the presence or absence of RD9, RD1 and RD4 by SybrGreenPCR and melting curve analysis, as previously described (14). Briefly, the detection and identification was divided into two reactions performed simultaneously. The first reaction was able to detect 16S rRNA (genus Mycobacterium), RD9 present (RD9+) for M. tuberculosis and RD1 absent (RD1-) for M. bovis BCG. In the second reaction, if RD4 absent (RD4-) was detected, the isolate was classified as M. bovis. In case that RD4 present (RD4+) was detected, the isolate was classified as member of the group of M. caprae, M.africanum, M. pinipedii or M. microti. During this study, Domogalla et al. (15) detected RD4- isolates of M. caprae. Considering this, the isolates were further identified by the presence of specific single nucleotide polimorfisms (SNPs) in the lepA gene by the previously described protocols (15, 16).
RESULTS
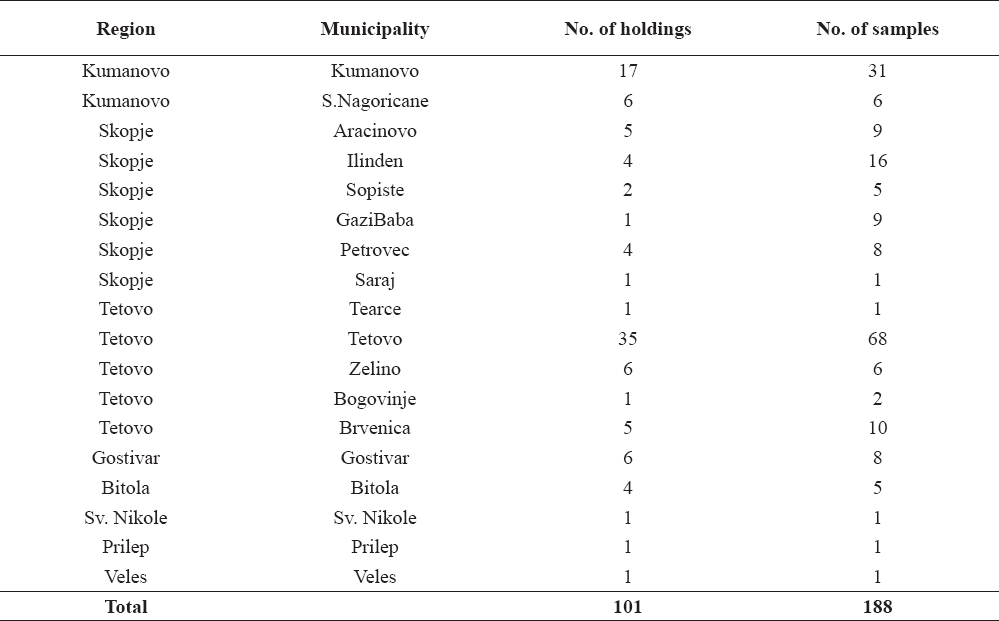
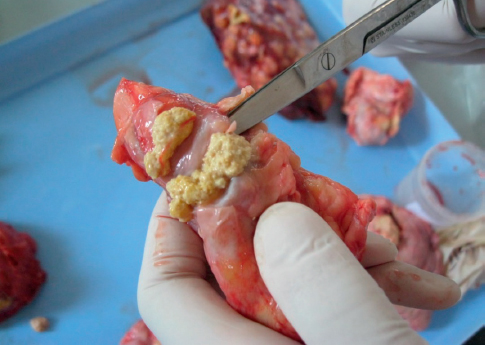
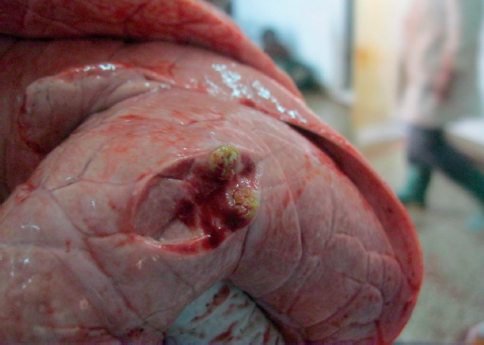
Typical tuberculous lesions (Fig. 1, 2 and 3) were detected in 62 animals (33%). Generalized bTB was diagnosed in 3 animals.
Figure 1. Caseous necrosis in a mediastinal lymph node
Figure 2. Tubercle in lungs
Figure 3. Multiple tubercles in lungs
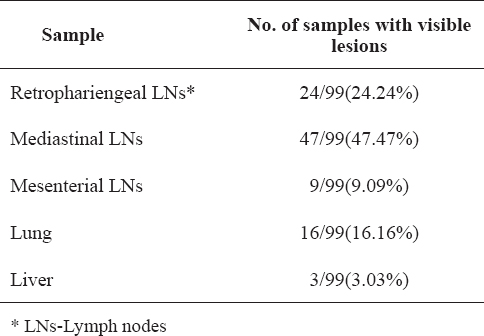
The distribution of the visible lesions from the sampled animals is shown in Table 2 and the prevalence of visible lesions per organ in Table 3. The highest prevalence of tuberculous lesions in the lymph nodes was 47.47%, detected in MdLNs (CI 95%: 37.63-57.31%) and the lowest was 9.09%, detected in the MsLNs (CI 95%: 3.43-14.75%).
Table 2. Distribution of visible lesions from the sampled animals
Table 3. Percentage of visible lesions per organ
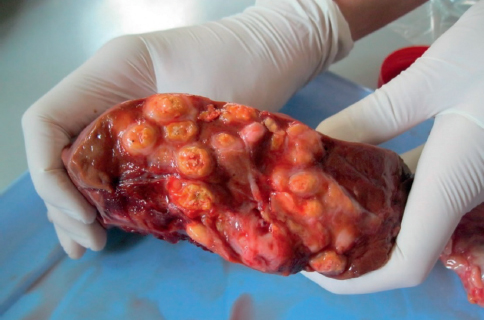
The bacteriological testing yielded 110 (59%) colonies with typical morphology for Mycobacterium spp. (Fig. 4). Following extraction, the conserved region in the 16s rRNA gene was amplified in all 110 samples with melting temperature (Tm) 79,50-81,60°C, thus assigning them in the genus Mycobacterium.
Figure 4. Suspect colonies on Stonebrink medium
RD9+ and RD1- were not present in any of the 110 samples which indicates absence of M. tuberculosis or M. bovis BCG. In twenty fours samples, besides the amplification of the genus control product, no RD specific amplicons were observed which points to the fact that those mycobacteria were not part of the MTBC and were classified as nontuberculous mycobacteria (NTM).
Seven samples were characterized as RD4+ group by giving positive result for RD4+ (Tm=77.70-78.90°C). In 79 samples, RD4- was amplified and by the melting curve analysis (Tm= 82.50-83.70°C), M. bovis was confirmed. The controls used in the study were M. tuberculosis H37Rv, M. bovis (supplied by the Croatian Veterinary Institute) and M. caprae (supplied by the Croatian Veterinary Institute). In each reaction, the three controls gave the species specific pattern for RDs.
In the RD4+ isolates, the specific SNPs in the lepA gene for M. caprae were amplified and resulted in confirmation of M. caprae. From the 79 RD4- isolates, specific SNPs in the lepA gene for M. caprae were amplified in 8 isolates.
In total, 71 isolates were characterized as M. bovis and 15 isolates as M. caprae. The Macedonian isolates of M. caprae were both RD4+ (n=7) and RD4- (n=8).
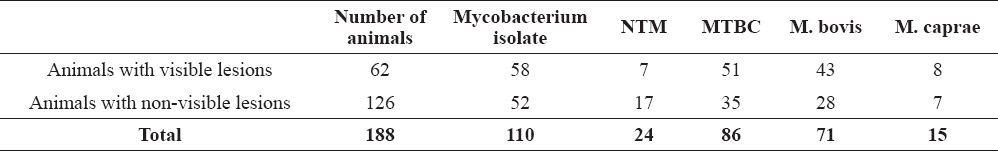
The prevalence of M. bovis isolated from the samples was 37.7% (CI 95%: 30.77-44.63%) and the prevalence of M. caprae was 7.9% (CI 95%: 4.04-11.76%). Mycobacterium bovis, M. caprae and NTM were isolated from both animals with visible lesions and animals with non-visible lesions (Table 4.)
Table 4. Mycobacterial isolates from animals with visible lesions and animals with non-visible lesions
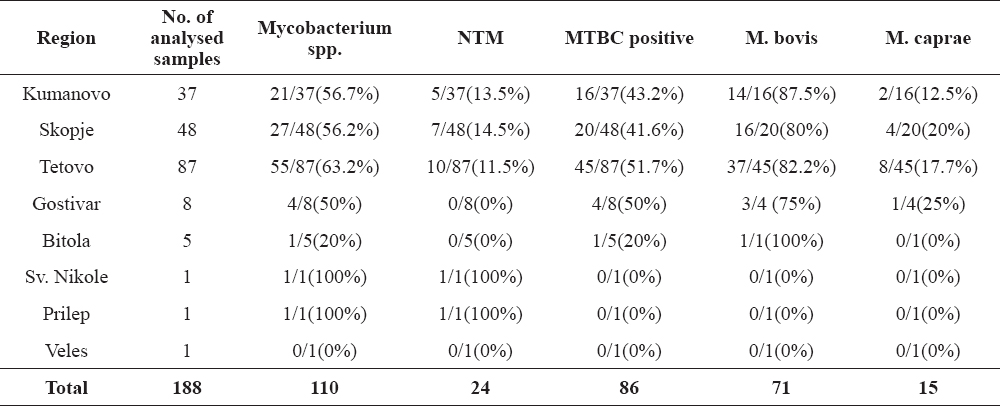
The detailed prevalence of M. bovis, M. caprae and NTM in the bTB positive regions in RM is shown in Table 5.
Table 5. Percentage of isolated M. bovis, M. caprae and NTM in the bTB positive regions
DISCUSSION
This study shows that bTB in the Republic of Macedonia is caused by M. bovis and M. caprae. According the data from the Food and Veterinary Agency of RM, bTB in RM during the research period (2011-2013) was diagnosed (by ICCT) in 0.1% of the tested cattle. The tuberculin skin test is the only diagnostic procedure that the National eradication program in RM relies on. The slaughterhouse surveillance is not performed successively to the slaughter of the reactors (17) which represents a considerable gap in the disease eradication. Slaughterhouse surveillance represents an important component in bTB diagnosis by detection of tuberculous lesions especially in commercial slaughtering and in animals not reacting in tuberculin tests in both Officially Tuberculosis Free countries (somewhere acting as the only bTB diagnostic tool) and nonOTF countries (17, 18).
During the slaughterhouse surveillance, we detected visible lesions in 62 animals (33%) and the prevalence of the lesions was highest in the MdLNs, followed by RLNs and lungs which indicates the respiratory route as a possible mode of infection. These results are similar with those observed by Fitzgerald et al. (19), where in adult cattle the typical lesions were predominantly located in the thoracic lymph nodes due to the lateral transmission via an aerosol route. Other authors have also registered the lungs and associated lymph nodes as locations were the tuberculous lesions were most prevalent (20, 21, 22). This comes from the fact that inhalation is believed to be responsible for 80-90% of all tuberculosis infections in cattle (20).
Besides the post mortem slaughterhouse surveillance, the laboratory methods (isolation and molecular identification) are not part of the bTB diagnostic and eradication scheme in RM. EU member states, besides the test and slaughter policy in the eradication programs, also implement laboratory investigations in terms of identification of the causative agents in order to confirm the disease and to evaluate the ante-mortem diagnostic methods (23, 24, 25). The isolation and identification of M. bovis and M. caprae in Spain is used to confirm tuberculosis infection in the herd, as well as for withdrawal of the status of herd free of tuberculosis (13).
The results from this research emphasize the need for continuous identification and typing of isolates in reactor animals in order to understand the disease epidemiology. The identification of the members of MTBC can be accomplished by a variety of molecular based assays that target specific regions in the mycobacterial genomes. The RDs or large sequence polymorphisms (LSP) are conserved in the mycobacteria belonging to the MTBC and due to their irrevocable character and intra-lineage consistency are widely used for construction of evolutionary schemes of the complex. (4, 26). The evolutionary scenarios suggest that the modern MTBC species evolved from a common ancestor through gaining these irreversible genomic deletions i.e RDs. (4, 27). The absence or presence of these genomic deletions can act as a discrimination tool for identification of the MTBC members – fact that has been used for designing and development of variety of PCR assays targeting the RDs. (14, 28, 29, 30). In our approach using the SybrGreenPCR and melting curve analysis as described by Pinsky and Banaei (14), we identified 79 isolates with RD4- and 7 isolates with RD4+. During our research Domogalla et al. (15) reported diversity in the RD4 in alpine M. caprae isolates and found three variants of the isolates: Allgau type (conserved RD4), Karwendel type (deletion of 5kb) and Lechtal type (deleted RD4). These variants were also confirmed in Bavarian and Austrian cattle and wildlife (red deer and fox) (31).
Differences in RD4 among M. caprae isolates were also reported in the study by Rodriguez et al. (32), where one isolate from a cow of Eastern European origin did not show presence or absence of RD4.
In order to identify whether the RD4- isolates were all M. bovis or the Macedonian M. caprae isolates have deleted RD4, all of the RD4- isolates were subjected to the protocol for detection of M. caprae specific SNPs in the lepA gene (15, 16). From the 79 (RD4-) isolates, 8 isolates were identified as M. caprae. The RD4+ isolates were all confirmed as M. caprae with this protocol. This confirms that the Macedonian M.caprae isolates are both RD4+ and RD4-. This illustrates that the deletions in RD4 detected by this protocol are not suitable marker for species differentiation of M. bovis and M. caprae in Macedonian M. caprae isolates. Besides the RD4 differences, these isolates also have shown differences in certain locuses in the MIRU-VNTR 12 loci typing panel (data not shown).
Mycobacterium bovis and M. caprae were isolated from 82.2% of the animals with visible lesions. The isolation of M. bovis and M. caprae for the diagnosis of tuberculosis in animals is usually considered as the golden standard (13). However, being unable to identify 100% of infected animals, this method cannot be considered as such even in samples with bTB lesions. The sensitivity of the method varies depending of different factors such as sampling procedure, type of samples, sample storage and implemented bacteriology protocols (type of decontamination, culture media) (13) and ranges from 4.7% to 85% (12, 33, 34, 35, 36, 37).
Mycobacterium bovis and M. caprae were isolated from 27.7% animals with non-visible lesions. The isolation of these species from animals with non-visible lesions further contributes to the fact that animals with non-visible lesions does not mean non-infected and non-infectious animals (38). Our findings correspond to the results from a research in Ireland where M. bovis was recovered from tissues taken from 19-28% of the animals with non-visible lesions (39). There are a variety of reasons for the lack of tuberculous lesions in infected animals, such as: early stages of infection, thoroughness of examination during slaughter, location and size of tubercles, prevalence of the disease and number of reactors in the herd (22, 39).
Nontuberculous mycobacteria were isolated from 13% of the animals with non-visible lesions. The culture-negative results could be because of early stages of infection, state of latency or non-specific sensitization in the tuberculin skin test due to infection with bacteria from non-mycobacterial origin (40).
The highest number of bTB agents were isolated and identified in the region of Tetovo, folowed by Gostivar, Kumanovo, Skopje and Bitola. Both of the mycobacteria were confirmed as causative agents of bTB in Croatia (41), Bosnia and Herzegovina (42), Spain, France, Germany, Italy, Slovenia, Austria, Hungary, Poland (43, 44), England (36), Algeria (45), China (46) and Ethiopia (47).
Over the years and according to the data from the Food and Veterinary Agency of RM (FVA), the northwestern part of the country i.e. the Tetovo region (Tetovo, Zelino, Tearce, Bogovinje and Brvenica) is the part with the highest prevalence of bTB. The persistence of the disease in this part of the country could be as a result of incomplete depopulation of the reactors in infected herds, lack of movement control, insufficient biosafety measures, inappropriate disinfection or inadequate repopulation of the holdings. This part of the country is bordering with Kosovo and Albania where transboundary movements without proper veterinary control could be an important issue that can contribute to the transmission of the disease between the neighboring countries. None of the neighboring countries of RM is an official tuberculosis free country. Therefore, strengthening of movement control would decrease the risk of the infection importation in the country.
Another aspect that should be taken into consideration is testing of other domestic and wild animals that can act as a true reservoir or a spill - over host for cattle as well as humans. This is due to the fact that M. bovis can affect a wide variety of species including domestic animals, pets and wildlife reservoirs (deer, wild boar and badger) (2).
Mycobacterium bovis and M. caprae are zoonotic pathogens which emphasizes the need for their diagnosis in the human population. In 2012, 25 EU member states reported 125 cases of human tuberculosis due to M. bovis (48). Mycobacterium caprae has also been identified as causative agent of human tuberculosis in Croatia, Germany, Spain and Austria (41, 44, 49). There are no official data for the presence of M. bovis or M. caprae in the human population of RM. According to the information from the National Reference Laboratory for human tuberculosis, the diagnosis is aimed to detect and not to identify a MTBC member, as well as to determine the antibiotic susceptibility of the isolate. Due to this, one can’t draw a conclusion about the zoonotic burden of M. bovis and M. caprae in RM. The fact that human tuberculosis is most prevalent in the northwestern part of the country (same as bTB), definitely draws attention to the need for further investigation and inter-institutional cooperation.
CONCLUSION
Our study demonstrates that M. bovis and M. caprae are causative agents of bTB in the Republic of Macedonia, isolated and identified from slaughtered reactor cattle with and without visible tuberculosis lesions. These findings highlight the importance of routine slaughterhouse surveillance and implementation of the laboratory diagnostic procedures for identification and typing of MTBC members as essential tools for a successful eradication program. Future research should be aimed to determine if Macedonian M. caprae isolates have varieties in the RD4 on which basis they can be subtyped, as well as to see if the obtained subtypes have a spatial distribution. Knowing the reservoir variety of the M. bovis and M. caprae, further research is needed in order to estimate the prevalence of the disease in other domestic and wildlife species.
CONFLICT OF INTEREST STATEMENT
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.