According to its chemical structure, nanoparticles are described as clusters of atoms that range from 1 to 100 nm in diameter (
1). Nanoparticlebased therapies in wound care are relatively new compared to traditional biomaterials that have been used for a long time (
2). Silver-nanoparticles (SNPs) (Nano-crystals) have been used effectively in the treatment of wounds in both emergency and cosmetic wards, for their effective antimicrobial activities, and ability in cytokine modulation and inhibition of inflammation (
3,
4). Therefore, they are also indicated for other skin injuries including small wounds, laceration wounds, abrasion wounds, and first and second-degree burns.
Historically, silver nitrate was used for the treatment of ulcers (
5). However, silver usage was reduced during the World War II when antibiotics were discovered and widely replaced the ancient schemes of antimicrobial approaches (
6,
7). Toward the end of the 20th century, the usage of silver for the treatment of wounds emerged again and silver nanoparticle products became commercially available and were frequently reported as being safe (
8,
9). It was reported that fibroblasts and keratinocytes had not been affected by the use of nano-silver (
10,
11).
Burns are a type of skin wounds classified into the following categories: 1. first degree, involving the epidermal layer; 2. second degree, involving the papillary layer and possibly the reticular layer of the dermis (
12); 3. third degree, including the epidermal layer and subcutaneous tissues (
13); 4. fourth degree, including damage to the full thickness of the skin and subcutaneous tissue in addition to the underlying supportive tissues along with the muscles, tendons, and ligaments (
14).
Skin wound healing includes overlapping patterns of several events including coagulation, inflammation, proliferation, matrix, and tissue remodeling. It is always expected for the wound to heal within minimum time, with less scarring, and paramount functional restoration of the injured site (
15).
One of the most important challenges in the treatment of burns is the infection because it delays the healing process by extending the inflammatory phase. Moreover, the presence of tissue debris at the site of the burn together with consumption of the body protein and immunosuppression may encourage bacterial growth (
16, 17). Recent evidence suggests that SNP have potent antiinflammatory effects (
18, 19, 20) and assist in the acceleration of wound healing (
21, 22).
Hence, the present study aimed to achieve treatment of third-degree burns by using pure SNP ointment (SNO).
MATERIAL AND METHODS
Animal experiments The study was conducted in the Medical College – University of Misan – Misan – Iraq. The skin burning procedure in the experimental animals (mice) was carried out according to the protocols approved by the bioethical committee (No. 7/19, 2018) which follows the guidelines of the Iraqi Ministry of Higher Education and Scientific Research for laboratory animal care and use.
Thirty-six (seven weeks old) laboratory male mice weighing 20-30 g were divided equally into three groups (n=12). They were grouped as: negative control (not burned and not treated), positive control (burned and treated with castor oil, and white petroleum jelly), and 3% SNO treated group (burned and treated with prepared ointment). Animals of each group were accommodated in a single cage for one week to achieve acclimatization, with free access to water and food. Animals were fed with standard commercial pellets. The accommodation was under controlled environmental conditions (temperature 23±2 °C, humidity 55±15%, lighting regimen of 12-h light: 12-h dark).
Preparation of 3% silver nanoparticles ointment (3% SNO)A homogenized ointment was prepared by mixing 1.5 g of silver nano-powder (Areej Al-Furat Company, Baghdad, Iraq) with 3 ml of castor oil (Turkey) and added to 45.5 g white petroleum jelly (Unilever, UK) (
23). A plastic spatula fixed to INGCO cordless drill was used to get a homogenized mixture. The maximum speed of the drill was 750 RPM according to the manufacturer’s instruction. The homogeneity of the ointment was evaluated by a scanning electron microscope (JEOL 7600, USA) (
24). The pH of the 3% SNO was measured with a pH-meter. One gram of ointment was taken and dissolved in distilled water at room temperature and stored for two hours. pH-meter was calibrated using 4 and 10 pH standards before use. The pH of the ointment was measured before each use (
23).
SpreadabilityThe technique described by Patel et al. (
25) was employed to test the spreadability. Shortly explained, 1 g of 3% SNO was placed on a glass slide which was covered by another slide, and a weight of 100 g was placed upon the upper slide for 5 minutes to evacuate the entrapped air and to form a thin layer of ointment between slides. The weight was removed and the excess material was scraped off. The weight was added again and the lower slide was held firmly while the upper slide was moving gradually. The time taken for the upper slide to separate away from the lower was recorded. This process was repeated three times and the average time was recorded.
Antibacterial activity of the 3% SNOThe antimicrobial activity of the 3% SNO against multidrug-resistant
Pseudomonas aeruginosa was evaluated by agar diffusion technique on the Mueller Hinton agar. The agar was prepared by following the manufacturer’s specifications. The bacterial isolate was cultured by the spreading method. The ointment was seeded in a bore which was previously made in the agar by using an 8 mm diameter sterile Cork borer. The dishes were allowed to stand at room temperature for 15 minutes to allow re-diffusion and then were incubated at 37 °C overnight. Bacterial growth was noted and the inhibition zone was measured (
26) and compared with the inhibition zones induced by the following antibiotics: penicillin, streptomycin, cefepime, amoxicillin, gentamycin, amikacin, levofloxacin, and imipenem.
Induction of full-thickness skin burnAnimals from the positive control and treated groups were anesthetized by intra-peritoneal injection of xylazine and ketamine (Alfasan, Woerden, Holland) in doses of 5, 100 mg/kg b.w., respectively (
27). The mice’s skin was shaved in the back region, and a full-thickness burn wound was induced with a metal plate (2x0.5 cm) heated to 100 °C. All animals were injected with 0.5 ml normal saline intraperitoneally to prevent dehydration. The burns were left uncovered without dressing throughout the study (
28).
Determination of LD50 and infective doseSix groups of laboratory mice (n=4) were used for a pilot study to determine the LD50 and infective dose. The first group served as negative control while the other groups were injected intraperitoneally with suspensions of
Pseudomonas aeruginosa in concentrations of 1010, 109, 108, 107, 106 CFU, respectively. Animals were observed for 10 days and the live and dead animals in each group were recorded to calculate the LD50. The infective dose was determined according to the equation of the LD50 (
29). Proportional distance = above 50% - 50% above 50% - below 50%.
Treatment of the burnsThree concentrations of SNO (1%, 3% and 5%) were assessed for effectiveness on skin burns healing. The 1% SNO showed little impact in the treatment of skin burns, the 3% SNO showed sufficient benefit on skin burns with no side effects, and the 5% SNO gave superior results but induced black pigmentations on the skin which was considered as unfavorable. Therefore, 3% SNO was used in the present study for the treatment of skin burns in experimental mice.
The 3% SNO was applied on the burnt skin in the animals of the treated group following 6 hours after burning. This treatment was repeated on every 8 hours for 14 days (
23).
Animals’ sacrifice and tissue harvestingFour mice of each group were sacrificed on the 3rd, 7th and 14th – day post burning of the skin, by deep anesthesia with chloroform (
30). The site of the burns was grossly inspected to confirm the changes and it was measured by Vernier calipers. An area of 2 cm2 of the skin, including the burned area, was harvested and placed in 10% neutral buffered formalin for histopathological study.
HistopathologyThe tissues were processed by ascending series of alcohol concentrations starting from 70 to 100%, cleared by xylene, and embedded with paraffin. Paraffin blocks were made and sectioned by microtome to 5 μm sections. Sections were stained with hematoxylin and eosin and inspected by a light microscope (
30).
Statistical analysisData were statistically analyzed by SPSS software statistical program. The data were expressed as the means and standard deviation. T-test was used. Values of p<0.05 were considered as statistically significant.
RESULTS
Homogeneity and spreadability of SNOScanning electron microscopic study showed the homogenized distribution of the SNPs within the ointment (
Fig. 1). The SNPs size in the ointment was confirmed as 20 nm. The spreadability of the SNO was 46 g.cm/sec.
 Figure 1.
Figure 1. Scanning electron micrograph shows the distribution of silver nanoparticles (SNP) in the ointment. Magnification 10000 KX
Antimicrobial activity of SNOThe ointment showed a 9 mm inhibition zone for the culture of
Pseudomonas aeruginosa corresponding to a panel of antibiotics which showed a range of inhibition zone between 0-2 mm for the same bacteria (
Table 1 and
Fig. 2).

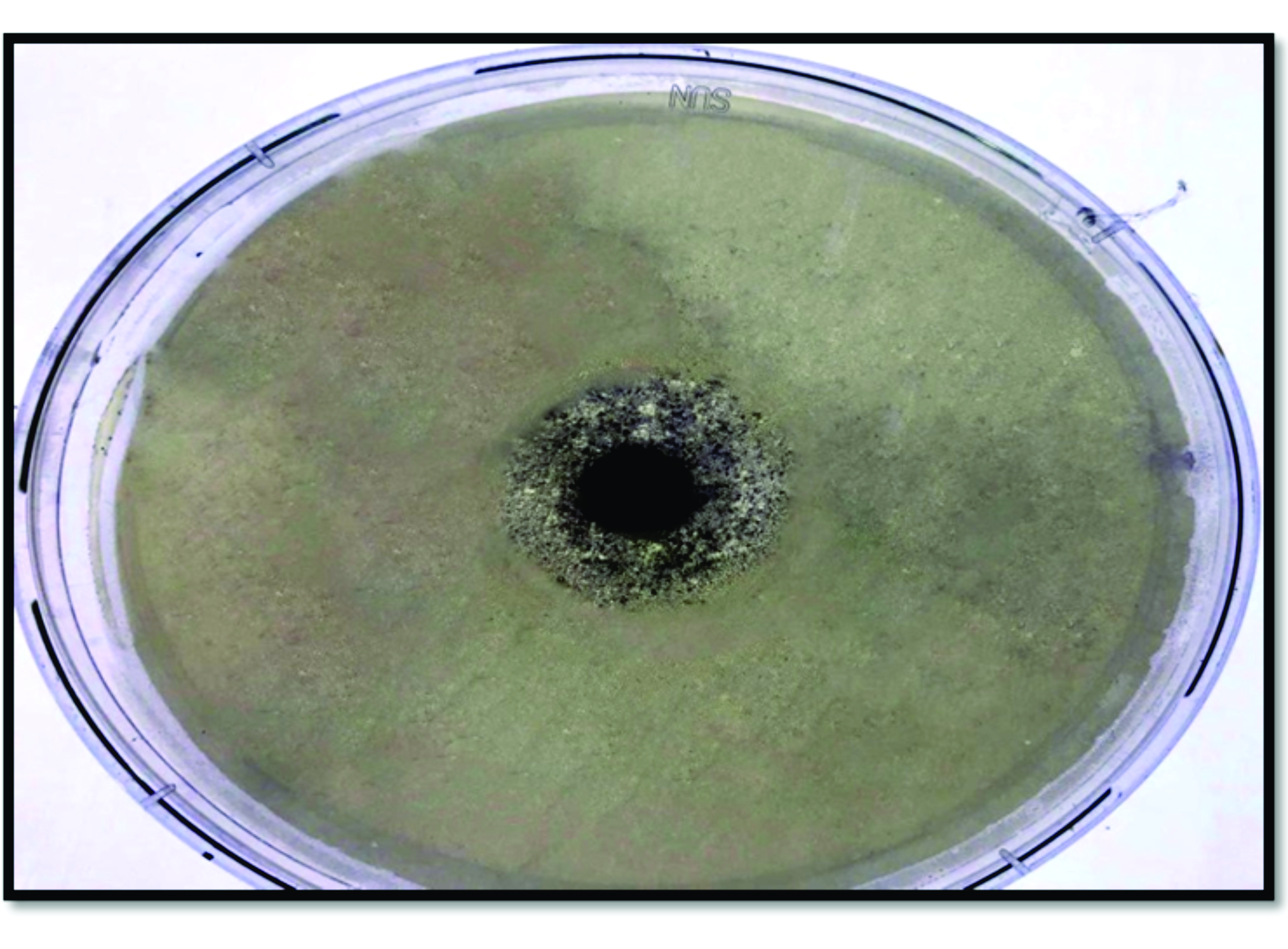 Figure 2.
Figure 2. Antibacterial activity of Nano-silver ointment against
Pseudomonas aeruginosaDetermination of LD50 and infective doseThe LD50 of
Pseudomonas aeruginosa was 109 CFU and the infective dose was 108 CFU. The later concentration was used for inducing infection in mice burns.
Animal studyGross lesionsGross inspection of the positive control revealed skin contraction and debris, 3 days after the burning of the skin. Marked scales were developed on the burned skin on the 7th and 14th day. The average size of the wounds of each subgroup on day 3, 7 and 14 was 1.98±0.10, 1.94±0.10, and 1.85±0.10 cm respectively.
In the SNO-treated group, scales were formed on day 3, but have sloughed off on day 7. The healing of the skin was almost completed by the 14th day, morphologically being similar to the skin in mice from the negative control group. Remarkable contraction of the burned skin was noticed in this group of mice which was 1.91±0.20 cm, 1.80±0.10 cm, and 1.50±0.30 cm on days 3, 7 and 14, respectively. The positive control group showed a limited contraction in the burned skin (
Table 2 and
Fig. 3).
 Figure 3.
Figure 3. Gross appearance of the burned skin in the positive control group with tissue debris throughout the experimental period: after 3 days (A); after 7 days (B); after 14 days (C). Treated group after 3 days (D); treated group after 7 days (E); treated group after 14 days (F)
HistopathologyHistological evaluation of the skin in the negative control group showed normal skin appearance where the epidermal layer contained stratified squamous keratinized epithelium and the adnexa in the dermal layer showed normal components (
Fig. 4).
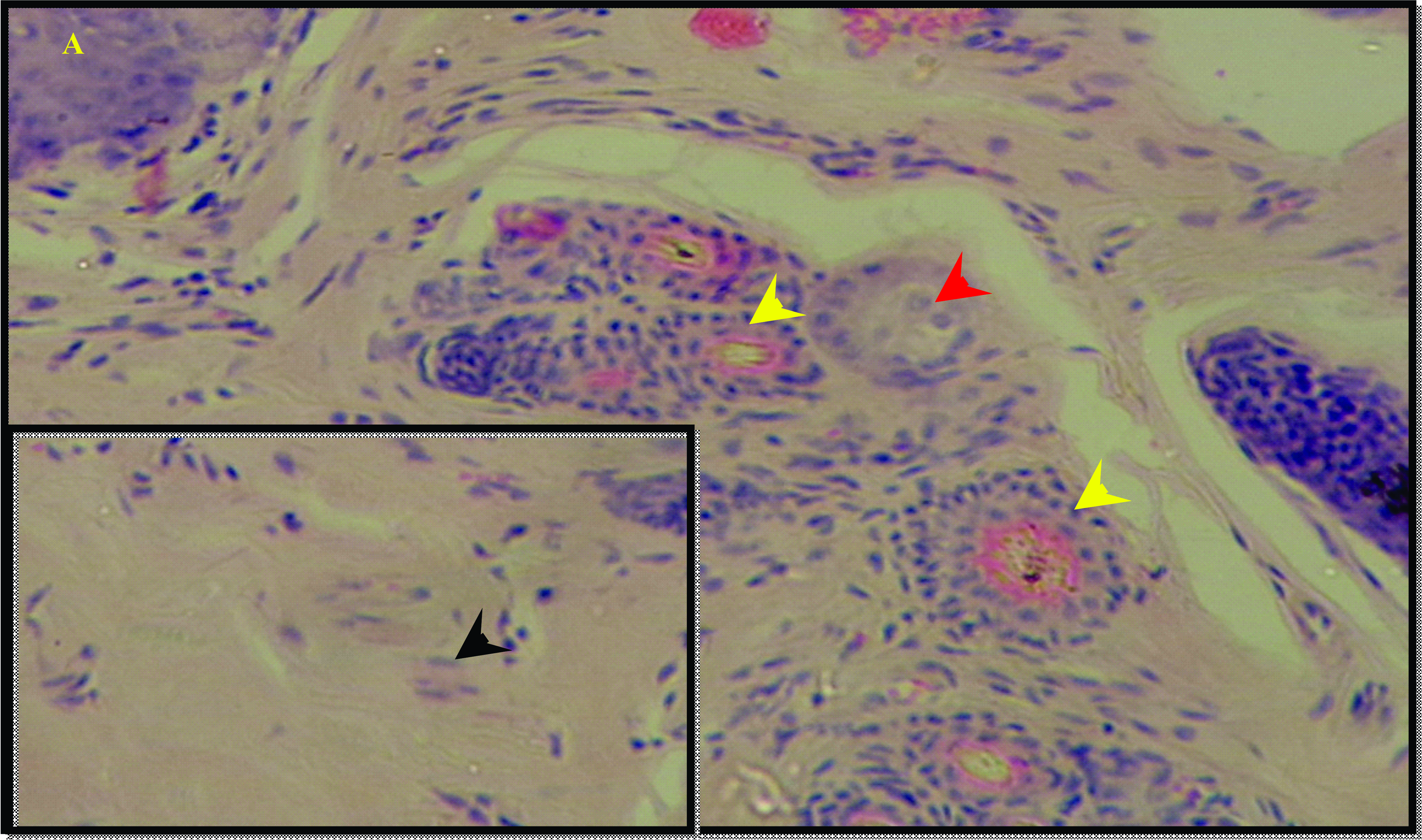 Figure 4.
Figure 4. Histological features of skin in control group revealed normal epithelium in the epidermal layer (black arrow head) and normal hair follicle (yellow arrow head) and sebaceous gland (red arrow head) in the dermal layer. H&E; x125 (A), x500 (B)
In both, the positive and SNO-treated groups, the entire thickness of the skin was affected. After three days, both the positive control and SNO-treated groups showed similar changes with alterations in the epidermal and adnexal layers (
Fig. 5,
6).
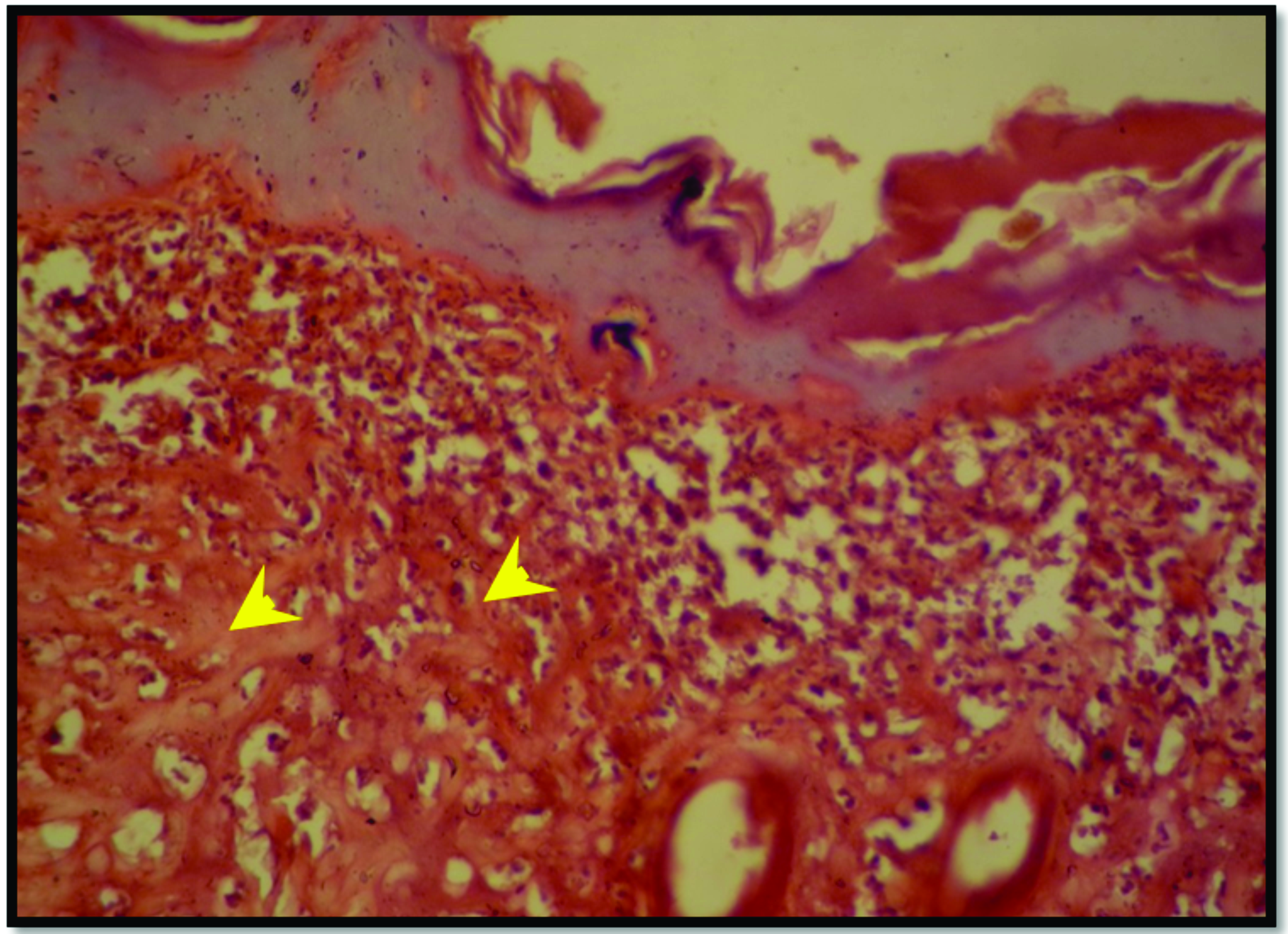 Figure 5.
Figure 5. Histopathological features of burned skin for the positive control group after 3 days, reveal necrotized epithelial cells of the epidermal layer (black arrow head), denaturation of the collagen in the dermal layer (yellow arrow head). H&E; x125
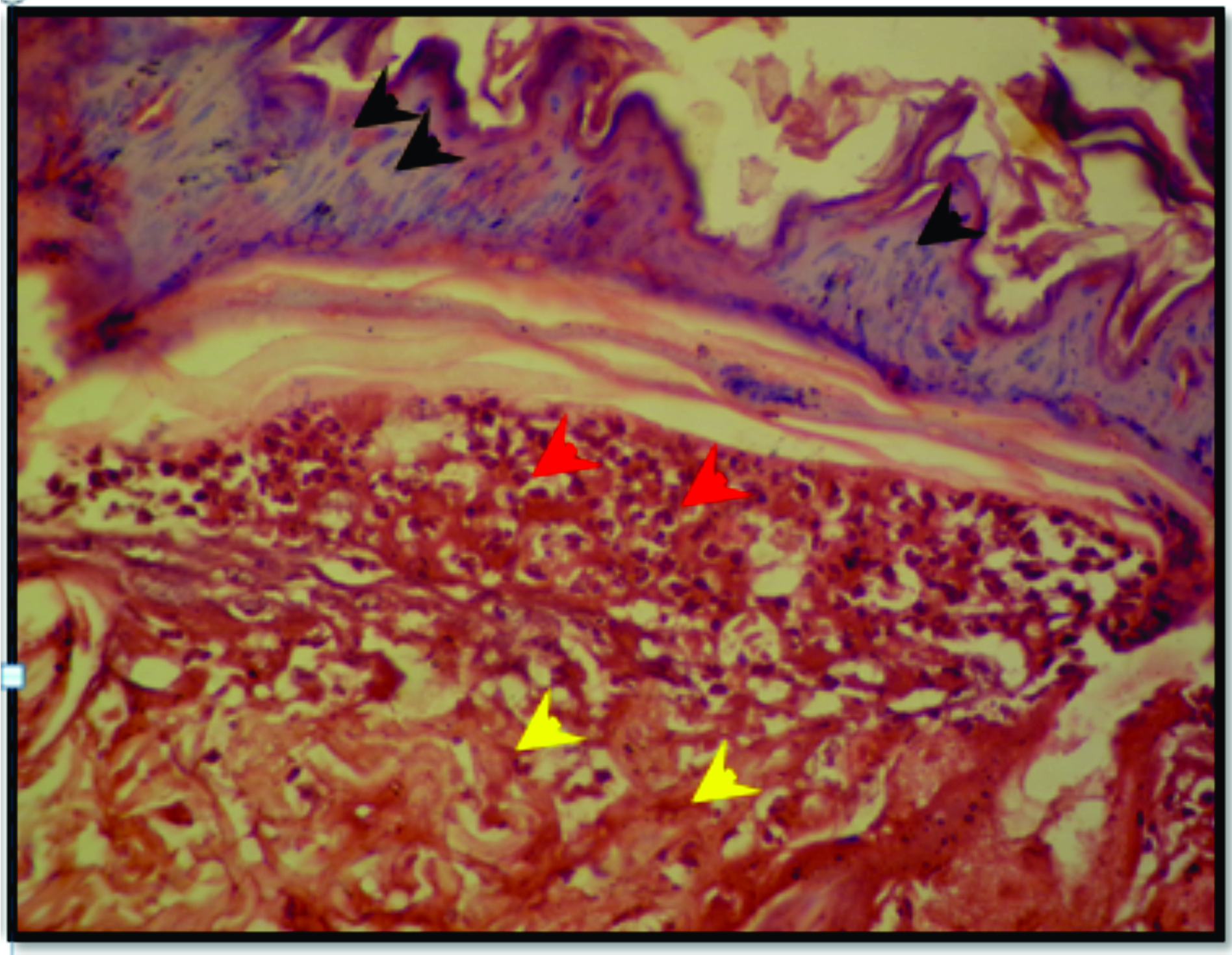 Figure 6.
Figure 6. Skin section from SNO-treated group after 3 days of burning reveal necrotized epithelial cells of the epidermal layer (black arrow head), denaturation of the collagen in the dermal layer (yellow arrow head) and neutrophilic infiltration subepidermally (red arrow head). H&E; x125
On the 7th day, the positive control group showed the accumulation of tissue debris in the site of the burn with an accumulation of inflammatory cells in the dermo-epidermal junction (
Fig. 7). These changes continued until the end of the experiment with slight epithelial regeneration in the epidermis and multiple abscesses in the dermal layer on the 14th day of the experiment (
Fig. 9).
In the SNO-treated group, after 7 days there was a reduction of the inflammatory infiltrate and marked epithelial regeneration bridging the burned site beneath the debris with the remodeling tissue in the dermal layer (
Fig. 8). On the 14th day, restoration of the full thickness of the epidermis was evident in addition to the marked remodeling of the collagen in the dermal layer with no hair follicles and sebaceous glands, and mild dermal scarring (
Fig. 10).
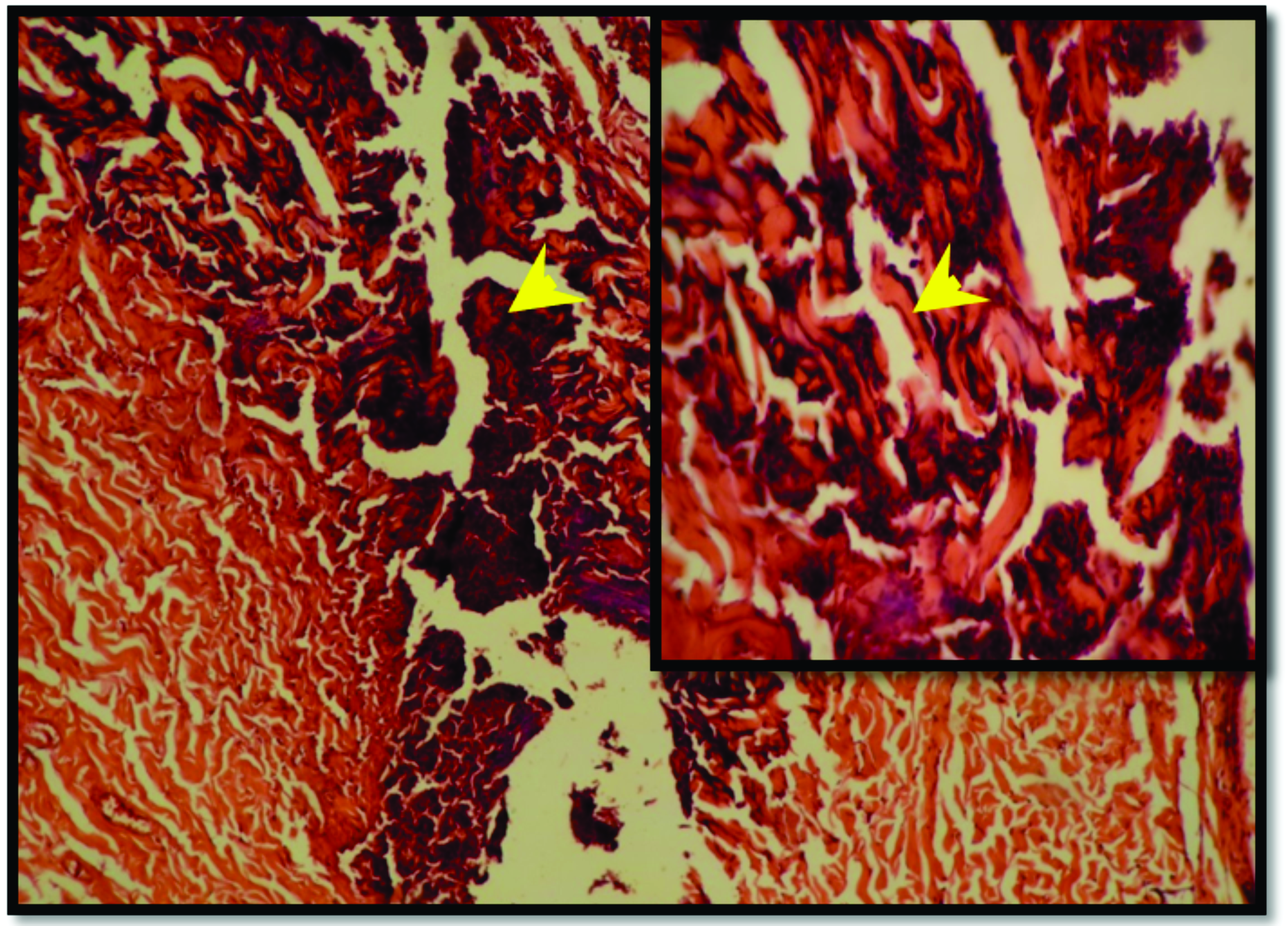 Figure 7.
Figure 7. Skin section of the positive control group after 7 days of burning shows tissue debris and denatured collagen (yellow arrow head). H&E; x125 (A), x500 (B)
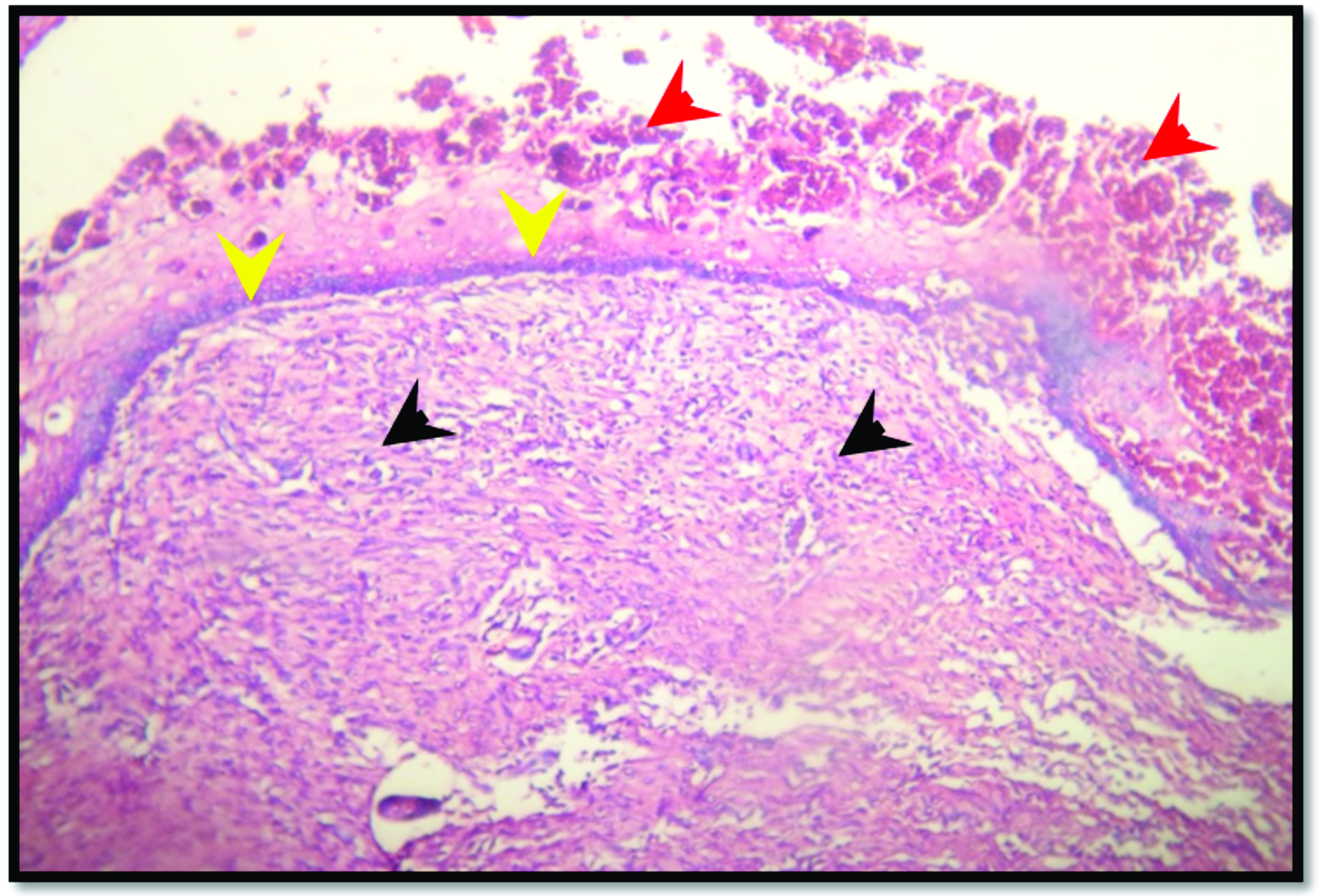 Figure 8.
Figure 8. Skin section from SNO-treated group after 7 days of burning reveals tissue debris in the superficial part of the skin (red arrow head), thin layer of newly formed epithelium beneath the debris (yellow arrow head), dense granulation tissue in the dermal layer (black arrow head). H&E; x125
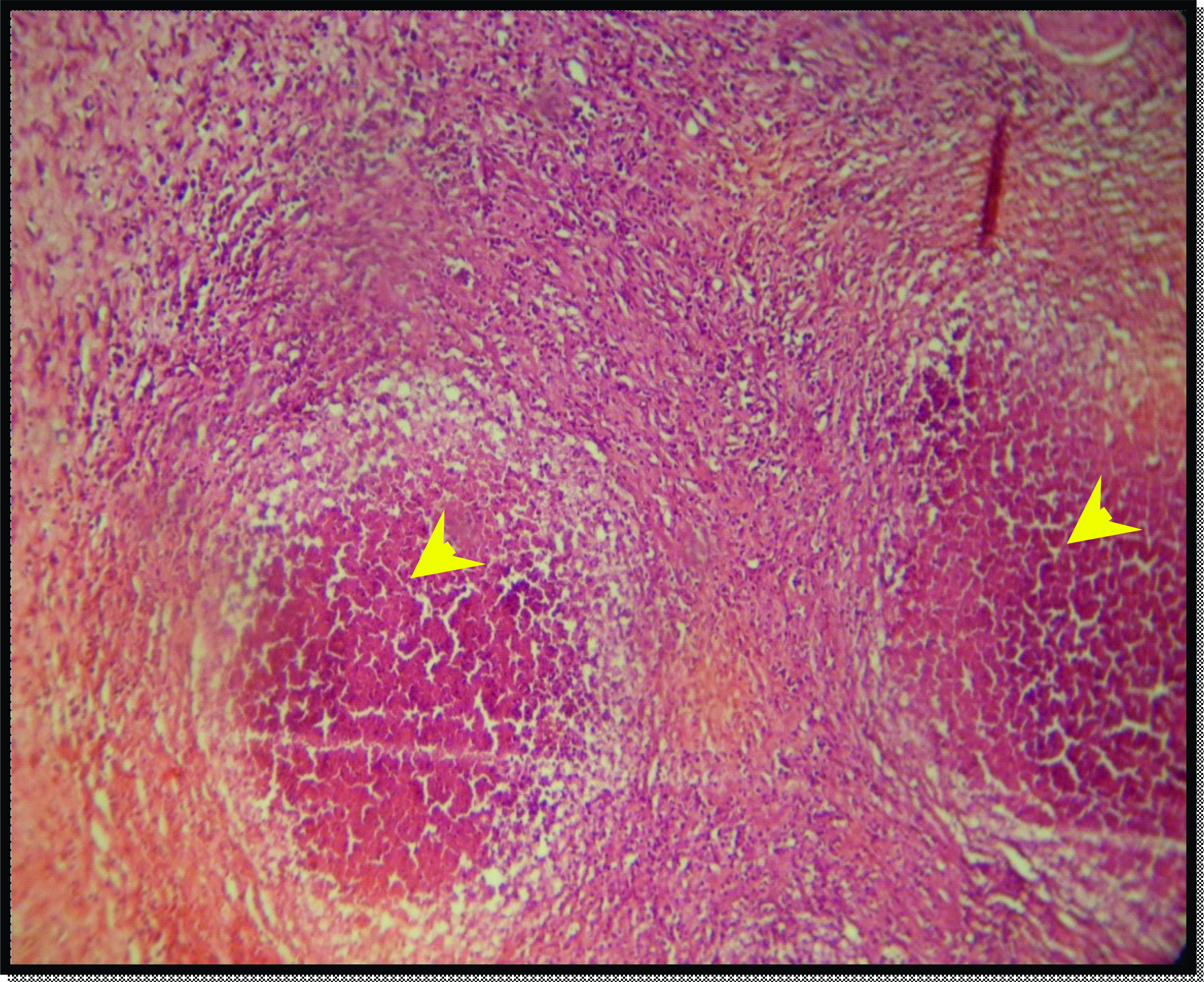 Figure 9.
Figure 9. Skin section of the positive control group after 14 days of experiment reveals abscess formation in the dermal layer (yellow arrow head). H&E; x125
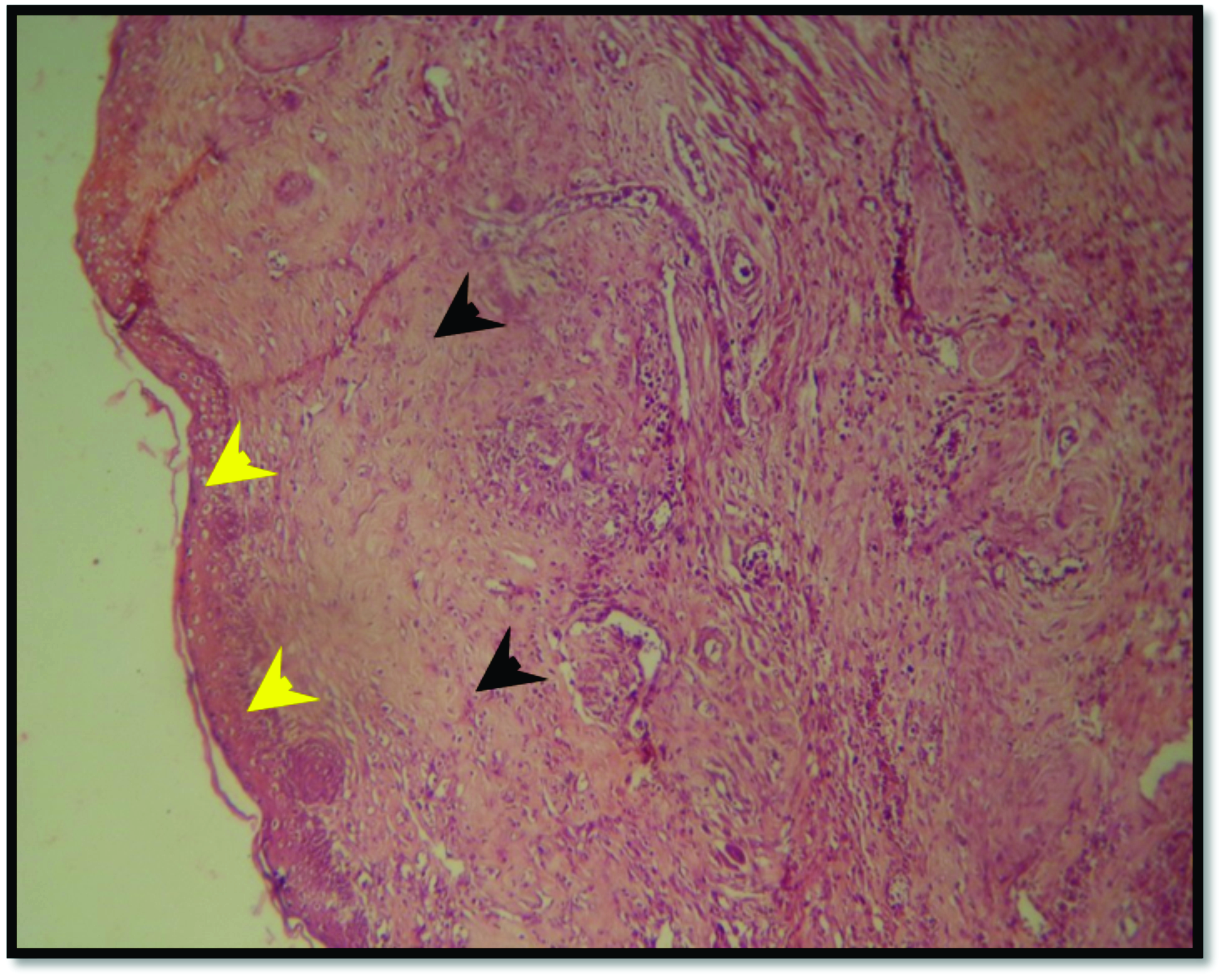 Figure 10.
Figure 10. Skin section from SNO-treated group after 14 days of burning reveals complete epithelial regeneration of epidermal layer (yellow arrow head) and mature collagen in the dermal layer (black arrow head). H&E; x125
DISCUSSION
Homogeneity and spreadability of the particles in the ointment were observed to ensure the pharmaceutical suitability of the product. The pH of the formulation was 6.8 which indicated good compatibility with skin. The results of the visual inspection for homogeneity of the material confirmed no lumps. The ointment homogeneity is important for proper application of the material to ensure even distribution on the affected area (
23).
Spreadability denotes the extent of the area to which the prepared formulations readily spread (
25). In the current study, the 3% SNO reported 46 g.cm/sec spreadability, which is negative correlation with the concentration and consistency of the gel (
24). Several factors affect the spreadability of each topical formula: 1) firmness, 2) shearing time, 3) affected site temperature, 4) viscosity, 5) evaporation rate of the vehicle and 6) active ingredient concentration that may also affect viscosity (
31). In the present study, the spreadability of the ointment was tested before its use to ensure appropriate application. The increasing of SNP concentration from 3-5% leads to adverse effects on application due to higher viscosity, whereas concentrations in the range of 1-3% is considered as appropriate (
32).
The 3% SNO which was used in this study showed marked inhibition zone in the agar media in comparison to various antibiotics which have been used in the medium. This is inconsistent with the other study which reported that silver interferes with bacterial cell wall and membrane of bacteria, and inhibits the respiration process (
33). Bacterial respiration is responsible for the release and capturing of chemical energy in the form of adenosine triphosphate. It is thought that the replication of the bacteria may be inhibited by SNP by various mechanisms, including bacterial DNA and membrane destruction through interaction with phosphorus-containing compounds and sulfurcontaining proteins, respectively (
34).
The current study revealed obvious elimination of the infection in the SNO-treated group. It is known that the phosphomannose isomerase is inactivated by silver. This particular enzyme converts the mannose-6-phosphate to fructose-6-phosphate, the latter being an important intermediate during the glycolysis. This represents the most common pathway in bacteria for sugar catabolism (
35). It has been confirmed that SNPs derived free radicals kill the microbes by inhibiting their growth and lowering its infectivity (
33).
The effect of 3% SNO in the treated mice from the experimental group was significantly different than the positive control group. The present study has showed improvement in the healing process that appeared on the third day after inflicting burns. At the end of the experiment, there was complete healing with marked regeneration of the epithelial layer in the epidermis of the skin, compared to the positive control group which showed augmentation in the inflammatory process and development of an abscess in the dermal layer. This may be related to the anti-inflammatory and antimicrobial activity of the silver that play an important role in the suppression of the bacterial load and the inflammatory response in the wound, hence lowering the tissue damage and stimulating the healing process. These findings are in agreement with another report that noted initial healing between the 2nd and the 7th day of the experiment with its peak effect on the 14th day in the treated animals (
36).
The anti-inflammatory activity of NSP was observed in both in vivo and in vitro experiments. The wound healing process includes the macrophages which play a vital role in the phagocytic ability, clearing the necrotic tissue, pathogenic microorganisms, and foreign matter. They can also secrete various cytokines and chemokines to stimulate cell proliferation and collagen deposition, promoting vascularization and granulation (
37).
In a standard wound healing pattern, the activation of macrophages in non-healing or infected wounds results in higher inflammatory response. Therefore, modulating the macrophage activation in such an instance would aid the healing process. It was clear in this study that there was no augmentation of the inflammatory state in the SNO-treated group. This suggests that NSPs have anti-inflammatory activity by regulating macrophage activity and inflammatory phase progression (
38).
SNPs are reported to inhibit the production of proinflammatory cytokines (
39) which is in agreement with the findings of the current study. A preliminary study also indicated that keratinocytes and fibroblasts produce growth factors (e.g., VEGF) (
40) and proinflammatory cytokines (e.g., IL-8, IL-6, IL-12 and TNF-α) that subserve inflammatory and immunological reactions to irritants. The SNPs may affect endothelial cells expression of the VEGF, which plays an important role in some pathological conditions, such as abnormal wound healing (
41).
CONCLUSION
The present study showed that SNO provided a good formula to treat skin burns through its antibacterial and anti-inflammatory activity, stimulating keratinocyte and fibroblast proliferation, enhancing collagen deposition. One of the limitations in the work was the small number of experimental animals for the various trials which included different dosages of SNO. Further investigations with larger animal number should be conveyed to additionally validate these findings.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest related to this article.
ACKNOWLEDGEMENTS
The authors would like to thank Assistant Prof. Dr. Mysaa Ghzi Jumaa the head of Department of microbiology for her support in accomplishing part of the work in the Microbiology laboratory, Medical college, University of Misan. Thanks also for Dr. Moammed Madi for his scientific advices regarding the bacteriological aspects of the research.
AUTHORS’ CONTRIBUTIONS
MAR performed the laboratory animals experiment and the pathological study, ANB supplied the instruments and equipment’s of the experiment, review and language editing of the manuscript. SBK provided the bacterial strain and accomplish the microbiological study of the study. HFA provided the pharmacological information and advices as well as calculation of the ratio of the contents of the 3% SNO.

 10.2478/macvetrev-2020-0032
10.2478/macvetrev-2020-0032











