The research study determined the effect of bovine whey supplementation in rats fed on high-fat diet on occurrence of myocardium damage and disfunction in its offspring. Eighty virgin female rats (Rattus norvegicus) (100-110 g body weight) were used for this study. Following mating, the pregnant rats were categorized into four groups: control, whey supplemented (W), high-fat diet (FD) and high-fat diet and whey supplemented group (FD+W). Whey supplementation alone or in combination with a high-fat diet was administered every other day during the gestation and lactation period. Offspring rats at the age of 1, 7, 14 and 21-day post-partum were sacrificed and their hearts were processed for histological, p53 immunohistochemistry, transmission electron microscopy and biochemical markers for cell damage. Offspring from the FD+W group exhibited improvement of the myocardium histological picture. Moreover, there was a lower accumulation of lipid deposits and regular organization of cardiomyocyte bands and intercalated discs. A lower p53 immune reaction and lower single strand DNA damage was noticed. The levels of the antioxidant enzymes (SOD and catalase) in the myocardium were increased, whereas the contents of IL6, MDA and caspase-3 were decreased, resulting in a reduction in inflammation and cell death. In conclusion, supplementation of whey to mother rats fed with high-fat diet alleviated the markers of cardiomyocyte injury in its offspring due to its antioxidant effect.
Bovine whey is a residual liquid of the processing of cheese, casein and yoghurt production with a pH of 5.9 - 6.6. It is composed of two main proteins, β-lactoglobulin (β-lg) and α-lactalbumin (α-la) which inhibit angiotensin-converting enzyme (ACE) and have antimicrobial, anticarcinogenic, and antihypercholesterolemic activity (
6). Whey is also containing many bioactive fractions including glycomacropeptide, β-lactoglobulin, α-lactalbumin, and lactoferrin, with numerous health benefits for cancer, infections, and inflammation (
7). Whey supplementation alleviated cardiomyopathy and lung inflammation which was associated with increased immunohistochemical staining of caspase-3, cyclo-oxygenase-2 and induced nitric oxide synthase in a high-fat diet mother rat (
8). There is no available research on the medicinal value of whey in the treatment of hyperlipidemia-related neonatal myocardial injury. The current research was undertaken to determine the alleviation role of bovine whey on the structural and functional components of offspring cardiomyocytes that had been damaged by a high-fat diet.
MATERIAL AND METHODS
The research was approved by the Ethical Committee of the National Institute of Health and Faculty of Science, Mansoura University, Egypt, and was conducted in compliance with the regulation for laboratory animal use. The official protocol number was Sci-Z-P-2021-44.
Preparation of a high-fat dietThe high-fat diet consisted of 20% lamb fat and other standard dietary components. The rats were being fed with this diet for four months before conception and during the entire time of gestation and lactation (
Table 1).
 Whey syrup supplementation
Whey syrup supplementationFresh bovine whey was daily provided from the Dairy Product Lab, Faculty of Agriculture, Mansoura University, Egypt. Each mother was administered with two doses of 2 ml by intragastric infusion, once at 8 AM and then at 2 PM, every other day. Whey syrup was administered according to the study carried out by Kandil et al. (
9).
Experimental workEighty virgin female rats (
Rattus norvegicus) (100-110 g body weight) were obtained from the Ministry of Health, Egypt, and were acclimatized. The zero date of gestation was noted by visualizing sperm in the vaginal smears in the next morning after mating. The pregnant rats were categorized into four groups (n=10): control, whey supplemented (W), high-fat diet (FD) and high-fat diet and whey supplemented group (FD+W). At 21 days-post-partum, the rats were fastened overnight and euthanized using chloral hydrate (300 mg/kg body weight) the following day. Maternal blood was collected at 10 AM, serum was separated by centrifugation, and then stored in refrigerator.
Light microscopyThe heart ventricles of 1-, 7-, 14- and 21-day-old offspring were fixed in 10% phosphate-buffered formalin (pH 7.4) and processed for histological investigations. Serial 5 μm thick sections were cut, stained with hematoxylin and eosin, and investigated under bright- field light Olympus microscope.
Transmission electron microscopyAdditional specimens were fixed in 2.5% phosphate-buffered glutaraldehyde (pH 7.4) and post-fixed in 1% phosphate-buffered osmium tetraoxide. The specimens were then dehydrated in higher grades of ethyl alcohol, cleared in propylene oxide, and embedded in epoxy resin. Ultrathin sections were cut with a LKB Ultratome IV and mounted on grids. They were stained with uranyl acetate and lead citrate, and visualized on a Joel 100CXl transmission electron microscope (Mansoura University, Egypt).
P53 immunohistochemistryFormalin-fixed and paraffin-embedded myocardial tissue sections (5 μm) were mounted on polylysine-coated glass slides and were kept at room 21- day old offspring were incubated with antibodies against p53 (Thermo fisher scientific, Fremont, CA, USA). The immune stained tissue sections were counter-stained with hematoxylin, investigated, observed, and then photographed under a brightfield light microscope. Image processing was performed at 40X objective of the digital camera fixed on the Olympus microscope with 1/2 X frame adaptor. The images were analyzed using Video Test software (Russia) on an Intel Core 5 based computer and the percentage area was calculated and reported.
Single cell gel electrophoresis (Comet assay)The myocardium of 21- day-old offspring were lysed with 1% sodium succinate, 2.5 M NaCl, 100 mM Na2EDTA, 10 mm Tris-HCl. The DNA was allowed to unwind after transferring to a buffer composed of 300 mM NaOH and 1 mM EDTA (pH 13). After 20 minutes of electrophoresis at 300 mA and 0.7 V/cm, the samples were neutralized with 0.4 M Tris–HCl buffer (pH 7). The slides were neutralized with tris buffer and stained with 100 μL of a 2 μg/ml ethidium bromide. The abnormal DNA strands lost their compact form and floated out of the nucleus into the agarose suspension with a low melting point. Fifty cells per each slide were analyzed using a Leitz Orthoplan (Wetzlar, Germany) fluorescence microscope with the aid of an automatic digital analysis system. Both tail length and DNA intensity were measured using image analysis software (
10).
Biochemical assaysMaternal rats’ serum was stored in a refrigerator. The heart tissue of 21-day-old offspring was homogenized in 10% ice-cold 2.5 mM-Tris buffer (pH 7.5) and centrifuged at 14,000 g for 15 minutes at 4 °C. The supernatant was stored in a refrigerator.
Total cholesterol (TC) (
11), triglycerides (TG) (
12), and high-density lipoproteins (HDL) (
13) were determined in the maternal serum. The low-density lipoproteins (LDL) were calculated from the total concentrations of cholesterol (TC), HDL-cholesterol, and triglycerides according to Friedewald et al. (14). The serum levels of leptin (CSB-E07433r), insulin (CSB-E05070r), adiponectin (CSB-E07271r) was determined by using ELISA kit of Cusabio Biotech Company (Huston, USA). Troponin I is determined by ELIZA Kit (ab246529), while alkaline phosphatase (ALP) (ab83369) and lactic dehydrogenase (LDH) (ab197000) was determined colorimetrically according to the instruction of Abcam Biotech Company (Cambridge, United Kingdom).
The heart samples of 21-day-old offspring were weighed, then homogenized in 10% ice-cold 2.5 mM-Tris buffer (pH 7.5) and centrifuged at 14,000 g for 15 minutes at 4 °C. The supernatants were stored in a refrigerator.
Superoxide dismutase was determined by a reduction of nitro blue tetrazolium (NBT) with superoxide radicals to a blue formazan color assayed colorimetrically at 560 nm (
15). Catalase was assayed by the degradation of hydrogen peroxide and the reaction product of non-reacted hydrogen peroxide was measured at 550 nm (
16). Malondialdehyde (MDA) was determined by measuring the thiobarbituric acid concentration (
17). Interleukin 6 was estimated by using Enzymelinked Immunosorbent Assay Kit of Cloud-CloneCorp, (catalogue No. SEA079Ra). Also, caspase-3, was determined colorimetrically by using a Stressgen kit (catalog No. 907-013) based on the hydrolysis of the peptide substrate acetyl-Asp-Glu- ValAsp p-nitroanilide (Ac-DEVD-pNA) and the resulting formation of the p-nitroaniline (PNA) was measured spectrophotometrically at 405 nm and compared with a standard curve.
Statistical analysisThe statistical analysis was performed by SPSS (version 13). Two-way ANOVA and post hoc analysis were employed between the control and experimental groups as well as between the high-fat diet and high-fat diet plus whey group. Data are presented as means ± standard error (SE). The t-test was calculated and considered statistically significant at p<0.05.
RESULTS
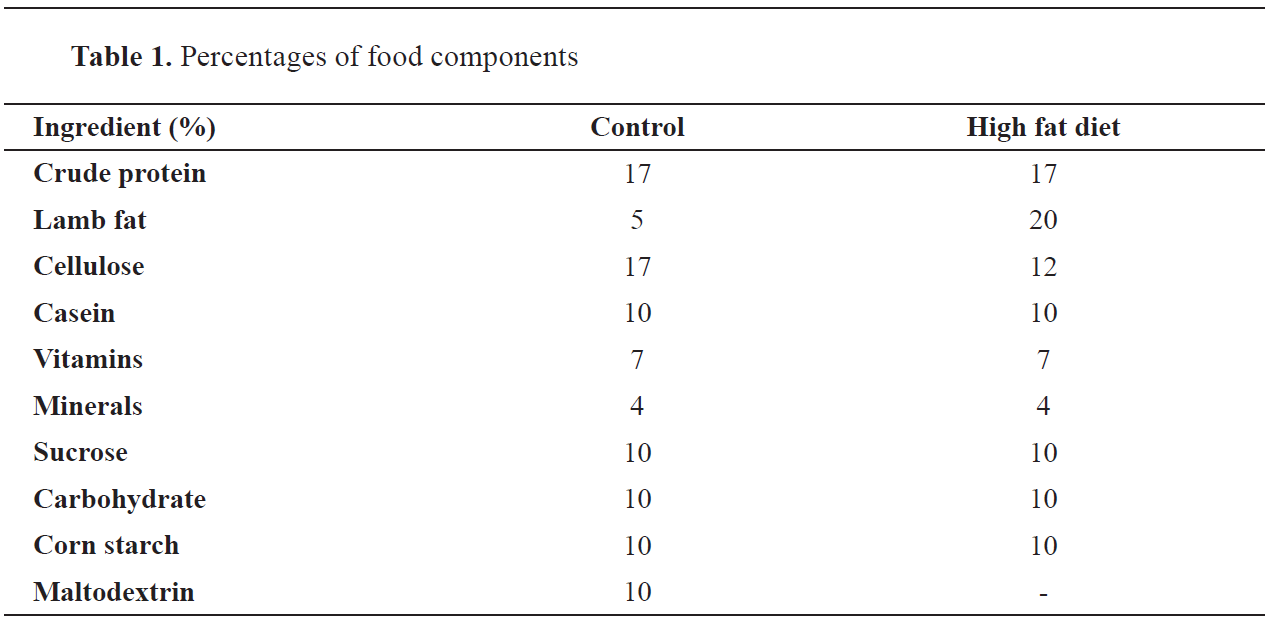
Body weight gainThe 1-, 7-, 14- and 21-day-old offspring from the FD group gained significantly higher body weight (6.00±0.75, 15.60±1.51, 23.00±2.95 and 34.20±4.49, respectively) compared to the control (5.58±0.47, 11.00±1.09, 15.50±1.35 and 21.80±1.73, respectively). The 1-,7-, 14 and 21-day-old offspring from the FD+W had insignificant increase in body weight compared to the control (5.80±0.54, 13.40±1.40, 21.20±2.93 and 30.00±2.71, respectively) (
Fig. 1).
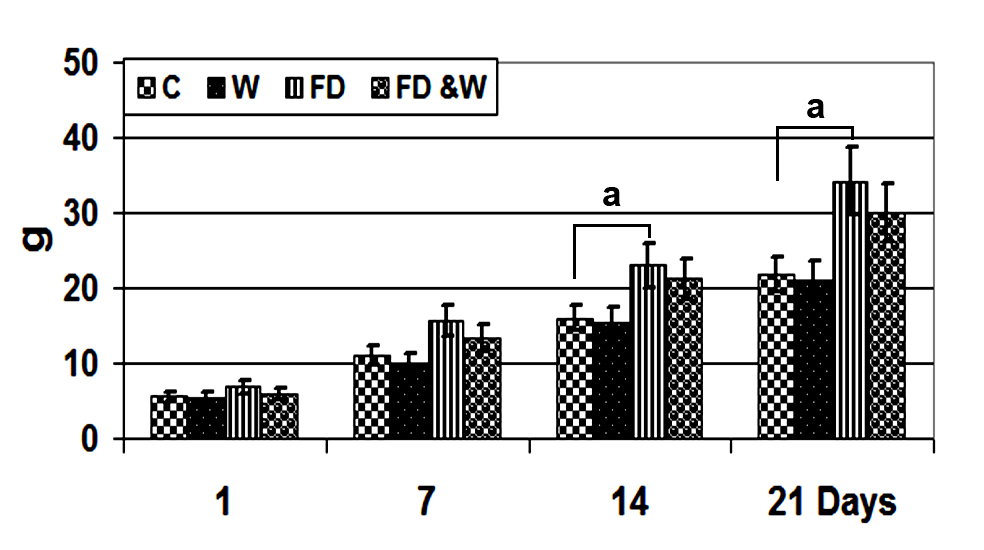 Figure 1.
Figure 1. Body weight gain of offspring from mother rats fed with a high-fat diet with or without whey supplementation. Each value is the mean ± SE (n=5/group). Abbreviations: C - control group; W - whey supplemented group; FD - high-fat diet group; FD+W - high-fat diet and whey supplementation group. The symbol ‘a’ marks significant difference of FD compared to control (P˂0.05)
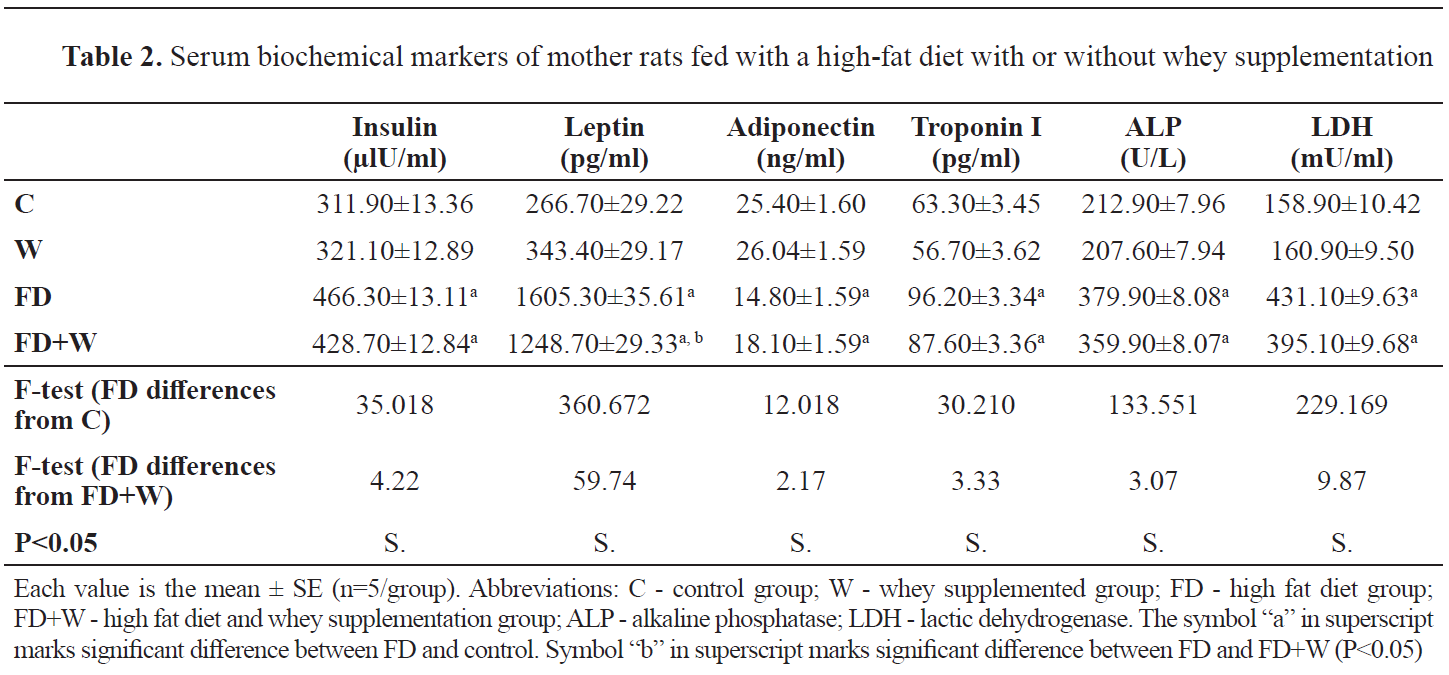
Maternal serum biochemical changesThe W group had non-significant lower serum levels of LDL, triglycerides, cholesterol and glucose (
Fig. 2). Whey administration has also reduced the rapid increase of insulin, leptin, troponin I, alkaline phosphatase and lactic dehydrogenase. Furthermore, mother rats of the FD+W showed decreased serum level of adiponectin (
Table 2).
 Figure 2.
Figure 2. Serum LDL, HDL, blood glucose, TC and TGL level of mother rats fed with a high-fat diet with or without whey supplementation. Each value is the mean ± SE (n=5/group). Abbreviations: C - control group; W - whey supplemented group; FD - high-fat diet group; FD+W - high-fat diet and whey supplementation group; LDL - low-density lipoproteins; HDL - high-density lipoproteins; TC - total cholesterol; TGL - triglycerides. The index "a" marks significant difference of FD compared to control at P˂0.05
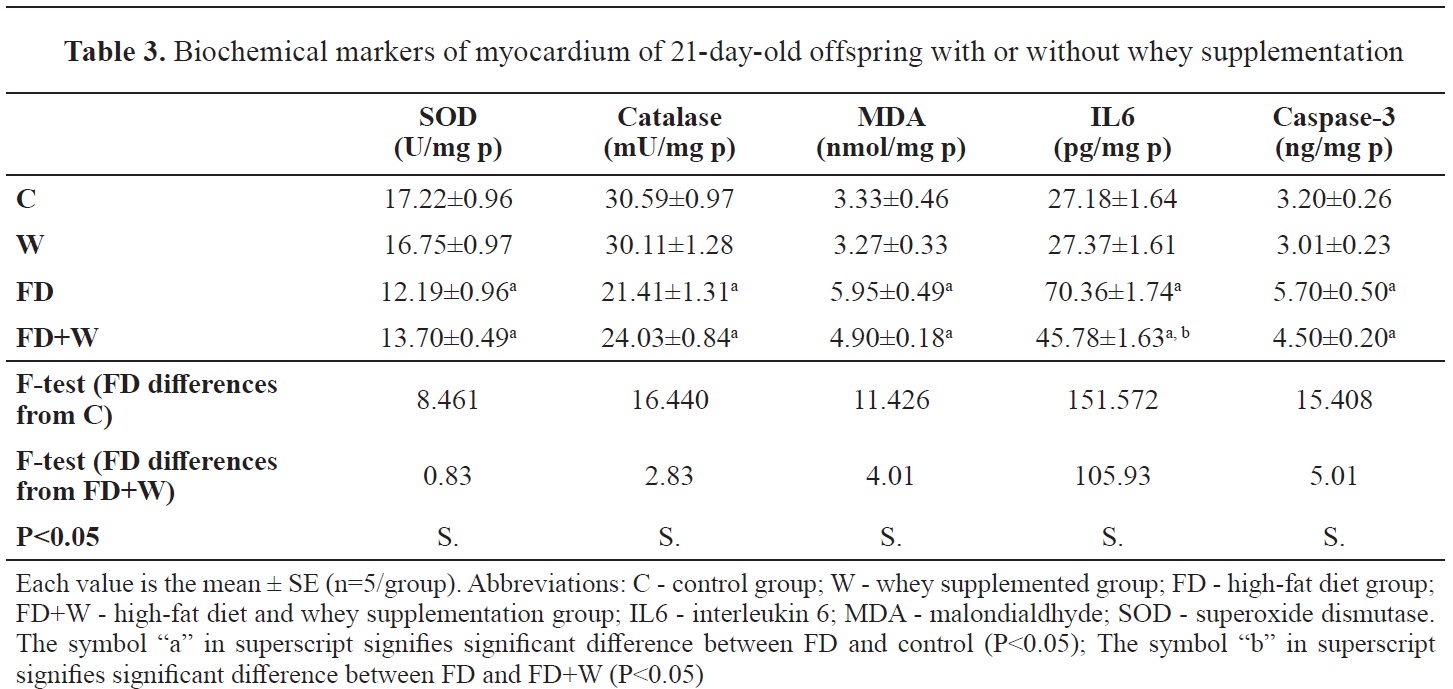
 Biochemical observations in myocardium of 21-day-old offspring
Biochemical observations in myocardium of 21-day-old offspringIn the FD group, the neonate myocardial tissues showed significantly lower SOD and catalase (12.19±0.96 and 21.41±1.31, respectively) compared to the control (17.22±0.96 and 30.59±0.97, respectively). The MDA content was significantly higher (5.95±0.49) than the control (3.33±0.46). In addition, the myocardial contents of IL6 and caspase-3 were significantly higher (70.36±1.74 and 5.70±0.50, respectively) than the control (27.18±1.64 and 3.20±0.26, respectively).
In the FD+W, there was a significantly lower MDA content (4.39±0.18) than the control (3.33±0.46). The level of IL6 was significantly lower (45.78±1.63) compared to the control (27.18±1.64). In addition, the caspase content had non-significantly higher (4.50±0.20) value compared to the control (3.20±0.26). The myocardial level of IL6 was significantly lower in FD+W (45.00±1.63) compared to the FD group (70.36±1.74). The other assayed parameters were nonsignificantly changed (
Table 3).
 Light and ultrastructural observation
Light and ultrastructural observationThe 1-, 7- and 21-day-old offspring showed disorganization of the myocardial fibers compared to the control (
Fig. 3, A-C). Focal leukocyte infiltration was observed between the muscle fibers. Numerous foci of necrotic fibers have been associated with hyalinization, fragmentation, sarcoplasmic vacuolation, and pyknosis (
Fig. 3 A1, B1, B2, C1 and C2). Offspring from HFD and W group had improved histopathological findings on the myocardial muscle (
Fig. 3 A2, B3 and C3).
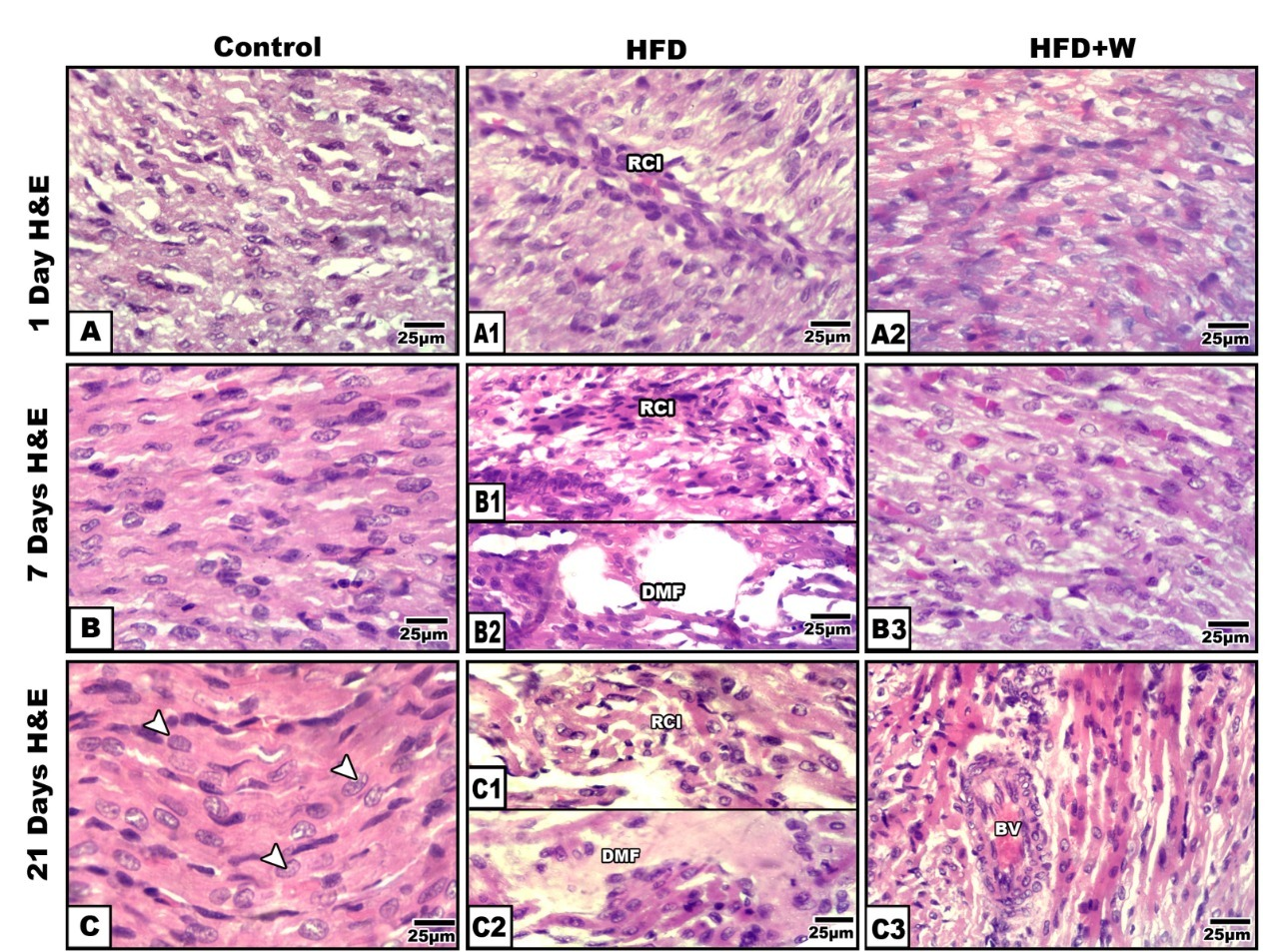 Figure 3.
Figure 3. Photomicrographs of histological sections of heart ventricle of rat offspring. 1 day-old offspring (A-A2); Control (A-C); 1- & 21-day-old offspring of mother fed with a high fat diet showing fragility of muscle fibers, hyaline necrosis and leukocytic infiltration (A1-B1&B2-C1&C2); 1-,7- & 21-day old offspring of high fat-diet mother supplemented with whey showing improved orientation of muscle fibers (A2, B3 & C3). H&E. Abbreviations: DMF - degenerated muscle fibers; RCI - round cells infiltration; BV - blood vessel; RCI - round cells infiltration; IH - internal hemorrhage
The 21- day-old offspring of FD mothers had irregular corrugated sarcolemma with pyknotic nuclei characterized by tightly compacted heterochromatin. The intercalated disks were abnormally folded. The interfibrillar mitochondria were atrophied and aggregated rather than being radially distributed among the fibrils (
Fig. 4 B-B2). Offspring of FD+W rats had improved muscle fiber. The muscle fibers had normal structure. The muscle bands were regularly oriented and both mitochondria and myocyte nuclear chromatin structures were more consistent (
Fig. 4 C-C2) compared to the regular and normal characteristic pattern of the control (
Fig. 4 A-A2).
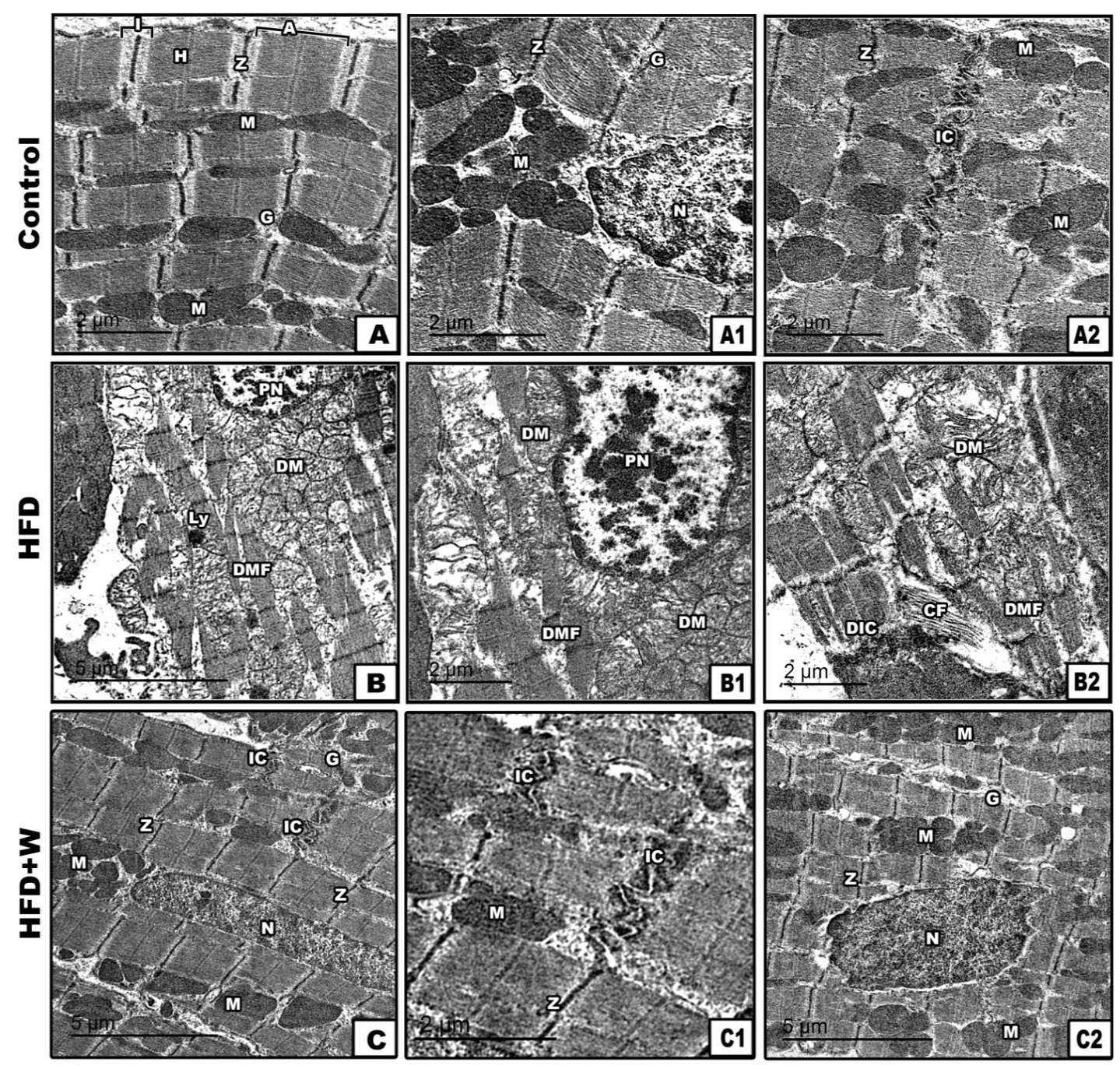 Figure 4.
Figure 4. Transmission electron micrographs of myocardium of 21-day-old rat. Control showing regular arranged muscle fibers with characteristic A-&I-band, Z-line & H-zone. Abundant mitochondria appears distributed in between muscle fibers (A-A2); 21-day-old rat from mothers fed with high fat-diet showing degenerated muscle fibers, damaged mitochondria, pyknotic myocyte nuclei (B) and degenerated intercalated disc (B-B2); 21 day- old offspring from mothers fed with high fat diet supplemented with whey showing ameliorated muscle fibers with regularly oriented muscle bands, cardiomyocyte nuclei and abundant mitochondria in between muscle fibers (C-C2). Abbreviations: CF - collagen fibers; DM - degenerated mitochondria; DMF - degenerated muscle fibers; DIC - degenerated intercalated disc; G – granules; H - H band; IC - intercalated disc; LY - lysosome; M - mitochondria; N - nucleus; PN - pyknotic nuclei; Z - Z-line
Immunohistochemistry of P53The myocardium of 21-day-old offspring from the FD group revealed overexpression of P53 compared to the offspring from the other groups (
Fig. 5 A-D). Hence, the FD group offspring had significantly higher immune reaction compared to the other groups (
Fig. 5 E).
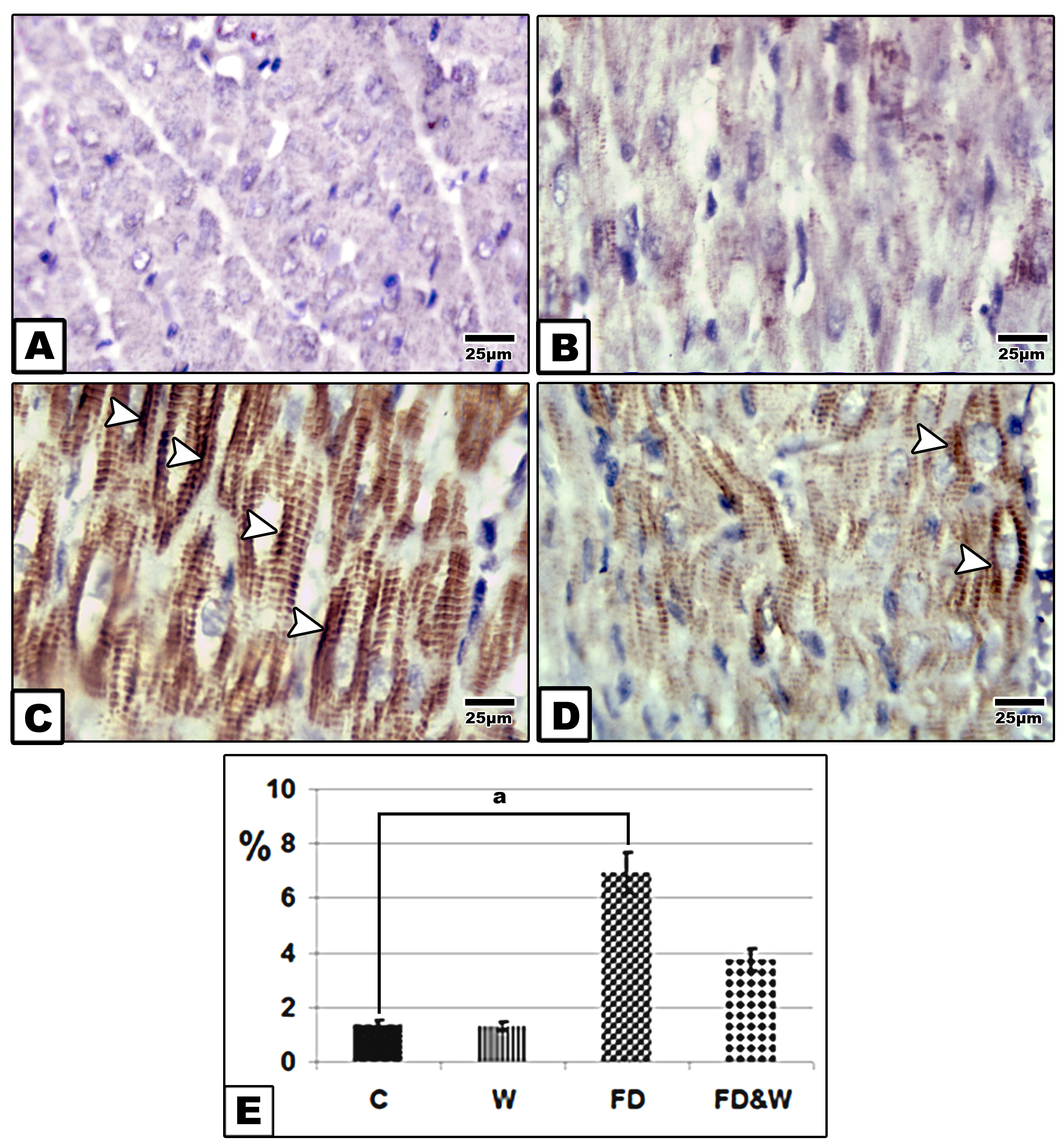 Figure 5.
Figure 5. Photomicrographs of formalin fixed histological sections of heart immunohistochemical stained with P53 of 21-day-old rat. Control and whey supplemented group showing decreased immunohistochemical reaction of P53 (A&B); Offspring of mother fed with a high-fat-diet group showing increase dark brown immunohistochemical reaction of P53 in muscle fibers manifesting cell death (C); Offspring of mother fed with a high fat-diet supplemented with whey showing decreased immunohistochemical reaction of P53 (D). Arrow head indicating reaction of P53. Histogram illustrating the percentages of immunohistochemical reaction expression of P53 in myocardium of offspring of mother fed with high fat-diet and improved in the group fed with a high fat diet and supplemented whey plus the fat diet (E). The symbol “a” marks difference compared to the control at P˂0.05
Single DNA fragmentation (Comet assay)Compared to the control, single strand DNA showed increased detached cardiomyocyte tail and DNA concentration reflecting DNA damage in 21-day-old offspring of mother ingested high-fat diet. However, those maternally supplemented whey plus fat diet showed marked decrease of DNA damage within the cardiomyocytes (
Fig. 6).
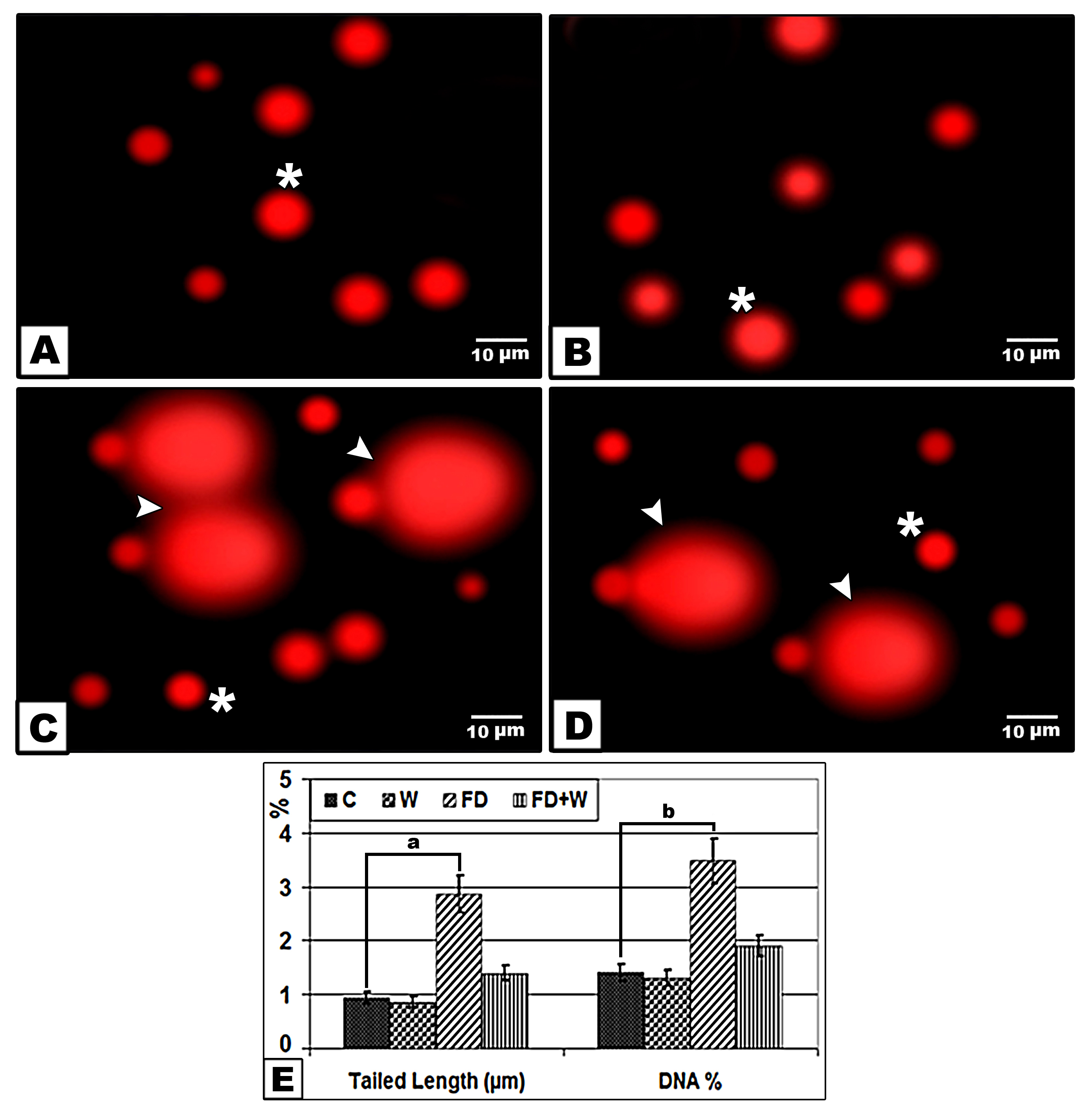 Figure 6.
Figure 6. Comet assay of heart ventricle of 21-day-old rat of mother from mothers fed with a high fat- diet with or without whey supplementation. Control (A); Whey supplementation (B); High fat-diet (C); High fat-diet supplemented with whey (D). The symbol (*) marks normal cells. Arrow head points detached tail region (DNA damage). Chart illustrating tailed length and DNA % of damaged cardiomyocytes (E). The symbols “a” and “b” mark significant differences between the high fat-diet and control (P˂0.05)
DISCUSSION
The current study revealed that offspring of FD mothers gained more body weight and showed symptoms of obesity compared to the offspring from mothers fed with normal diet. This trend was first noticed at parturition and continued throughout the suckling period. These findings are conflicting with the report of Ribaroff et al. (
18) stating that maternal high-fat diet resulted in increased body weight only at weaning. Butruille et al. (
19) reported that the increased body weight of rat offspring from obese mothers was a result of increased visceral adiposity.
In addition, histopathological abnormalities were detected on the myocardium of the FD offspring. The changes were characterized by hyalinized necrosis of the cardiomyocytes associated with the dense leukocyte cell infiltration and disorganized muscle fibers. At ultrastructural level, the distorted sarcolemma was characterized with almost missing Z and I bands, deformed intercalated discs and the presence of pyknotic nuclei. The interfibrillar mitochondria were atrophied and clumped instead of being radially distributed between the fibrils. Interfibrillar lipid deposits have been clearly identified. The current results are consistent with the findings of Mdaki et al. (
3) who reported impaired cardiac structure and function in rat offspring from mothers fed with high-fat diet. Guzzardi et al. (
20) has reported a thicker left ventricular posterior wall and disrupted diastolic contraction in pig neonate from obese mothers.
Mother rats fed with a high-fat diet had higher serum levels of LDL, triglycerides, total cholesterol and blood glucose that may predict early signs of diabetes. This was highly indicative of myocardial damage in offspring and could be predictive to the onset of diabetes. The increased oxidation of fatty acids and release of free radicals (
21) leading to maternal myocardial fibrosis (
8) could be attributed to the significantly higher serum levels of troponin1, LDH and ALP which was highly predictive of cardiotoxicity in the mothers. This is compliant with the report of Magalhães et al. (
22) on diabetic rats fed with high-fat diet.
The cardiomyocyte damage of offspring from FD mothers was characterized by significantly lower antioxidant enzymes (SOD and catalase) resulting in oxidative stress (high MDA levels). It was found that this led to increase the overproduction of the superoxide (O2−) and oxygen species from the damaged interfibrillar mitochondria. The same was also reported for myocardial tissues of rats fed with a high-fat diet (
23).
The high expression of P53 in cardiomyocytes of FD offspring was also reported by Attia and Taha (
24) in apoptotic cells of diabetic rats. These findings were confirmed by the comet assay which detected increased myocardial caspase-3. Rat offspring from mothers fed with a high cholesterol and triglyceride diet were more likely to develop type 2 diabetes (
25). It is also known that troponin-I is associated with managing of the actin thin filament within muscle cells. Its function is carried out in conjugation with the alkaline phosphatase. The increased activities of both enzymes indicated cardiomyocyte damage (
26). You et al. (
27) reported that the elevation of alkaline phosphatase predicted cardiovascular disease.
The FD+W group offspring had regular cardiomyocyte structure which is coinciding with the lower biochemical markers for cardiomyocyte damage. Increased levels of the myocardial SOD and catalase coincided with the decreased oxidative stress. Also, whey was found to decrease the IL6 which facilitated myocardial inflammation, and caspase-3 and troponin-I involved in the cardiomyocyte cell death. Whey supplementation was found to improve the histological structure and function of aging cardiac tissue (
28) by regulating the reninangiotensin- aldosterone system (
29), insulin levels in mice (
30) and by increasing cardiac levels of the antioxidant enzyme activities of GPx and GSH in cardiomyocytes (
31). In addition, high-fat diet was found to decrease the gut microbiota in mother rats (
32). Administration of probiotic alleviated the reduction of triglycerides, cholesterol and LDL of eighteen-month-old
Macaca fuscata offspring from mothers fed with a high-fat diet (
33).
CONCLUSION
Whey exhibited antioxidant activity and contained adequate amounts of protein and lactobacilli. Supplementation of whey with a high-fat diet facilitated upregulation of serum levels of adiponectin and alleviated the markers of cardiomyocyte injury. Whey supplementation improved the antioxidant defence and reduced the oxidative stress in the myocardium of rat offspring from mothers fed with high-fat diet mother.
CONFLICT OF INTEREST
The authors declared that they have no potential conflict of interest with respect to the authorship and/or publication of this article.
ACKNOWLEDGEMENTS
This research was supported by the Faculty of Science, Mansoura University, Egypt.
AUTHORS’ CONTRIBUTIONS
HIEl-S designed the experiment, formatted the data, revised, and constructed the manuscript. HAEl-G constructed the first manuscript version, performed the histopathology investigations, and statistical analysis. EME have performed the experimental work and prepared the histopathology and transmission electron microscopy specimens.

 10.2478/macvetrev-2022-0017
10.2478/macvetrev-2022-0017








