Fully-grown mammalian oocytes contain morphologically evident but transcriptionally inactive nucleoli called “nucleolus-like bodies” (NLBs). These nuclear structures are essential for early embryonic development. Removing oocyte NLBs (enucleolation) before their activation leads to developmental failure, mainly at the time point of embryonic genome activation. The present study examined the developmental and expression dynamics of embryos derived from intra- (pig) and interspecies (mouse) nucleolus (NLB) transferred porcine oocytes after parthenogenetic activation. Activation of rDNA transcription and pre-rRNA processing in NLB re-injected embryos was observed by real-time qRT-PCR analysis, targeting the expression rates of 45S rRNA and levels of 18S rRNA. Reinjection of NLBs from mouse or porcine GV oocytes into the enucleolated MII stage porcine oocytes supported the embryonic development up to the blastocyst stage after their activation. Intra- and intergeneric NLB transferred embryos demonstrated rDNA transcription initiation at the 8-cell stage, corresponding with the embryonic genome activation in porcine parthenogenetic embryos (control). The measured levels of 18S rRNA in these experimental embryos showed delayed initiation of pre-rRNA processing at the blastocysts stage compared with the control (8-cell stage). However, the porcine embryos with re-injected mouse NLB displayed significantly higher levels of 18S rRNA than the control and experimental intraspecies group. In conclusion, NLBs from different mammal species (mouse) can enhance the quality of enucleolated porcine oocytes and thus support their early embryonic development after activation.
The major genome activation (MGA) in mammalian embryos is unambiguously characterized by consecutive activation of RNA-polymerase-I, -II, and -III transcription, resulting in successful embryonic development. Processes of rDNA transcription, mediated by RNA-polymerase-I, and the subsequent processing of rRNA to the formation of pre-ribosomal subunits, are localized in the nucleolus. The activation of rDNA transcription is also reflected at the ultrastructural level by the transformation of the dense nucleolar structure (nucleolus precursor body - NPB) into a functional tripartite nucleolus consisting of fibrillar centers, a dense fibrillar component, and the granular component (
1). In contrast to MGA, rRNA synthesis increases during oocyte growth and peaks at the beginning of follicular antrum formation in early mammalian embryos. The full meiotic competence coincides with a markedly decreased transcriptional activity and formation of temporary compact fibrillar nucleolar sphere (nucleoluslike body - NLB) in nearly grown oocytes at the germinal vesicle (GV) stage (
2). Immediately before germinal vesicle breakdown (GVBD), the NLB disappears and the chromatin condenses to form an irregular network of individual bivalents (
3). Despite the high similarity between nucleoli of GV oocytes and transcriptionally active embryonic cells, the condensed fibrillar spheres, i.e. NLBs in oocytes and NPBs in early embryos, exhibit slight differences. Tracing the material of oocyte NLBs material during the maturation and early post fertilization stages shows that they are briefly existent in zygotes and early embryos, where the nucleolus is formed
de novo in transcriptionally active embryonic cells (
4). The key role of maternal nucleolus in embryonic development has been characterized in more detail, especially after the invention of a new method for microsurgical removal of NLBs (enucleolation). Its’ reimplantation into oocytes and early embryos at different maturation or developmental stages has been reported with variable findings even between different animal species (
5, 6, 7). These experiments unambiguously confirmed the importance of NLBs during normal development of mice and pig embryos, regardless of the method of their preparation (parthenogenetic activation,
in vitro fertilization, or SCNT) (
8, 9).
We hypothesized that NLBs and embryonic NPBs might support the embryonic development after parthenogenetic activation of oocytes previously subjected to interspecies nucleolar transfer. The current study aimed to elucidate the processes of MGA associated with the transcription and processing of rRNA during the development of interspecies (mouse/pig) nucleolus transferred embryos by reinjecting the NLBs from mouse or porcine GV stage oocytes into the previously enucleolated and matured porcine MII stage oocytes. Additionally, the study aimed to determine the embryonic development, timing, and intensity of
de novo rRNA synthesis following the parthenogenetic activation of microsurgically manipulated versus non-manipulated porcine oocytes.
MATERIAL AND METHODSChemicals and mediaUnless indicated otherwise, all chemicals were purchased from Sigma Chemical Co. (Prague, Czech Republic).
Ethical standardsAll experiments in this research were conducted by the ethical standards of the relevant national regulations on the care and handling of farm and laboratory animals and the EU Directive 2010/63/EU on the protection of animals used for scientific purposes. The experimental protocol was approved by the Institutional Research Ethics Committee (permit no. 2547/19-312/4).
Collection of porcine oocytesPorcine ovaries were collected at a local abattoir and transferred to the laboratory in saline buffer at 30-35 °C. After triple washing with PBS supplemented with 0.01% polyvinyl alcohol (PVA), the presumable GV-stage oocytes were aspirated from 3-6 mm follicles (
10). The cumulus-oocyte complexes (COCs) were washed three times in M2 medium. COCs were
in vitro maturated in bicarbonate-buffered medium 199 supplemented with 4 mg/mL of bovine serum growth proteins, 0.5 μg/mL FSH, 0.5 μg/mL LH, 40 μg/mL sodium pyruvate, 70 μg/mL L-cysteine and 50 μg/mL gentamycin (Gibco Invitrogen, Prague, Czech Republic) (IVM) for 30 min at 38.5 °C and 5% CO
2 in humidified atmosphere. Subsequently, the cumulus cells were completely removed by gentle pipetting. Denuded oocytes were used for further enucleolation procedures.
Collection of mouse oocytesMouse ovaries were collected from 6–10-week-old ICR female mice (Anlab, Prague, Czech Republic), euthanized by a cervical dislocation. To obtain the GV stage oocytes, females were treated with 5 IU equine chorionic gonadotropin (eCG; Intervet International, Boxmeer, Holland) and after 44 hours, the GV oocytes were isolated from large antral follicles using a sharp needle. COCs were briefly pre-cultured in maturation medium (αMEM) supplemented with bovine serum albumin (BSA, 4 mg/mL), gentamicin (50 μg/mL), Na-pyruvate (20 mM) and dbcAMP (150 μg/mL) at 37 °C under an atmosphere of 5% CO
2. The oocytes were denuded from the surrounding follicular cells by vigorous pipetting.
Enucleolation of porcine and mouse GV oocytesThe porcine and mouse GV stage oocytes were enucleolated (ENL) according to a method described elsewhere (
11). Briefly, GV stage oocytes were manipulated with a micromanipulator in M2 medium supplemented with 7.5 μg/mL cytochalasin B/D and 0.1% PVA (Enu-M2). After brief centrifugation (5 min at 1,350 g), porcine oocytes were transferred into 10 μL droplets of Enu-M2 covered with paraffin oil. For ENL, the enucleolation pipette was inserted through the
zona pellucida and slightly attached to the GV membrane surface. Subsequently, a gentle suction was applied on the pipette tip to translocate the nucleolus through the GV membrane. The enucleolated porcine oocytes were cultured until MII stage in IVM medium, as described above. The nucleoloplasts (nucleoli surrounded by plasma membrane) were temporarily kept in Enu-M2 medium before the re-injection into previously enucleolated oocytes.
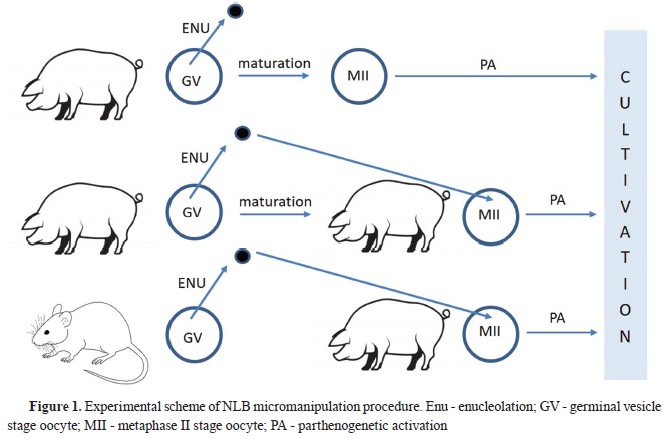
Nucleolus re-injection and parthenogenetic activationThe enucleolated porcine MII oocytes were re-injected with recently isolated porcine (P+P) or mouse (P+M) nucleolus, as described previously (
Fig. 1) (
6). The nucleolus reinjected and intact (parthenogenetic control) porcine MII oocytes were cultured in porcine zygote medium-3 (PZM3) supplemented with 3 mg/mL BSA (PZM3-BSA) for 4 hours at 38.5 °C, 5% CO
2, and were then parthenogenetically activated. The oocytes were transferred into a 5.5% mannitol solution and activated by a single DC pulse of 1500 V/cm for 100 μs (CF-150; BLS, Budapest, Hungary). The activated oocytes were washed at least three times in PZM3-BSA medium supplemented with 5 μg/mL of cytochalasin B to prevent the exclusion of the second polar body. Cytochalasin B treated oocytes were washed in PZM3-BSA medium and cultured for 6 days. The embryos of experimental and control groups were collected at the zygote phase, 12 hours post activation (hpa), 2-cell (22 to 24 hpa), 4-cell (46 to 48 hpa), 8-cell (70 to 72 hpa), and blastocyst stage (140 to 144 hpa). They were processed for real-time qRT-PCR analysis.
Real-time qRT-PCRThe genes analyzed in the present study are crucial in the process of ribosome biogenesis organized in a nucleolus where the transcription, maturation, and assembly processes are performed.
In order to uncover the role of oocyte NLB on the initiation of rRNA transcription and processing, the levels of
de novo synthesised 45S rRNA and processed 18S rRNA were observed during development of embryos acquired by parthenogenetic activation of intact (control) and nucleolus exchanged oocytes.
All the pools were done in triplicate and contained 3 embryos after parthenogenetic activation of nucleolus exchanged and intact oocytes, each at zygote, 2-cell, 4-cell, 8-cell, and blastocyst stages.

Before RNA isolation, 2 pg of rabbit globin RNA (Gibco, Invitrogen, Carlsbad, CA) was added to the samples as an internal standard. The total RNA was extracted using the NucleoSpin RNA XS kit (Macherey-Nagel), and DNase I treatment was performed directly in the column as described by the manufacturer. Subsequently, reverse transcription was carried out using 2.5 μM random hexamers (Applied Biosystems, CA) and MuLV Reverse Transcriptase (Applied Biosystems, Foster City, CA) in total volume of 20 μL for 1 hour at 42 °C.
Real-Time PCR was performed on ABI 7500 Fast Real-Time System using Power SYBR® Green PCR Master Mix (Applied Biosystems, CA) containing MgCl
2, dNTP, and AmpliTaq Gold® DNA polymerase. Specific primer sequences for the detection of
de novo synthesized 45S rRNA and processed 18S rRNA were designed as described in
Table 1.
StatisticsAll
in vitro culture and qRT-PCR experiments were repeated more than three times. The maturation and developmental competence of oocytes and embryos in each group was analyzed by Fisher’s exact test and the chi-square test. Mean values of qPCR data within each group (P+P, M+P, and parthenogenetic control) were analyzed by oneway analysis of variance method using SigmaStat 9.0 software (Systat Software Inc., San Jose, USA). Significant results from SigmaStat 9.0 were further analysed by Tukey test. Results from both experiments were considered significant at p<0.05.
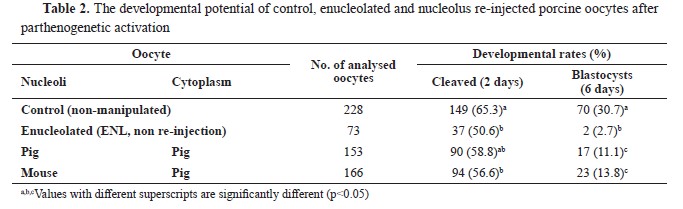
RESULTSDevelopmental competence of nucleolus exchanged oocytesENL oocytes showed lower (p<0.05, 50.6%, 37/73 oocytes) cleavage rate than the control (intact, 65.3%, 149/228 oocytes,
Table 2). Furthermore, the blastocyst rate of the ENL rapidly decreased to 2.7% (2/73 oocytes) which was significantly lower (p<0.05) than the control (30.7%, 70/228 oocytes) and experimental groups (11.1%, 17/153, and 13.8%, 23/166 oocytes, respectively) (
Table 2).
After the re-injection of porcine NLBs into the porcine enucleolated MII stage oocytes, the reconstructed embryos partially regained the capability to reach the blastocyst stage (11.1%, 17/153 oocytes). The MII stage porcine oocytes re-injected with mouse NLB displayed very similar cleavage rate (56.6%, 94/166 oocytes) with the intraspecies group (58.8%, 90/153 oocytes). Moreover, 13.8% of these embryos reached to the blastocyst stage (
Table 2).
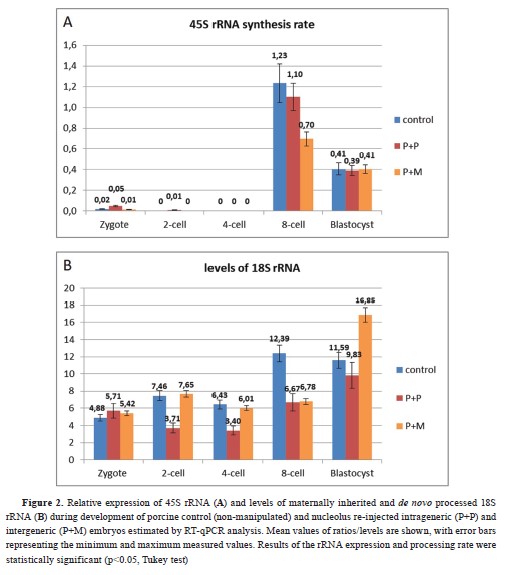
Initiation of rRNA transcription and pre-rRNA processingThe synthesis of 45S rRNA and 18S rRNA was detected at the 8-cell stage in the control group (
Fig. 2a, 2b). Further qPCR analyses of 45S rRNA synthesis rate showed that intra- and interspecies nucleolus re-injected embryos initiated lower
de novo rRNA production at the 8-cell stage compared with the control (
Fig. 2a). The processing of pre-rRNA in both experimental groups was initiated at blastocyst stage. Moreover, the porcine embryos with re-injected mouse NLB displayed significantly higher levels of 18S rRNA in the blastocyst stage compared with the control and experimental intraspecies groups (
Fig. 2b).
 DISCUSSION
DISCUSSIONThe remodeling of fully functional nucleolus during major embryonic genome activation is closely related to the activation of rDNA transcription, pre-rRNA processing, and
de novo synthesis of ribosomes (
2, 13). It is already known that NPBs are involved in the regulation of chromatin reorganization and formation of chromocenters with regulating potential for transcriptional silencing during early embryogenesis (
9, 14). The importance of oocyte NLBs and embryonic NPBs in the above-mentioned processes was demonstrated in recent experiments where these structures were microsurgically removed from oocytes or zygotes and were subsequently re-injected at different time points of their maturation or development, using different animal species (mouse, pig) (
15, 4).
In the present study, the enucleolation and nucleolus reinjection procedures were used to elucidate the importance of oocyte NLBs in development of porcine parthenogenetic embryos. Moreover, the study investigated the possible positive effects of interspecies nucleolar transfer on the developmental potential of formerly enucleolated porcine oocytes. The cleavage rate of parthenogenetically activated enucleolated porcine oocytes after the first two days was 50.6%. Only a fraction of the embryos reached the blastocyst stage (2.7%). Similar results were obtained in other studies where most of the fertilized or parthenogenetically activated mouse enucleolated oocytes, matured to MII phase, failed to cleave to the blastocyst stage. On the other hand, the developmental competence of mouse oocytes (MII) regained after the reinjection of NLBs from GV stage oocytes, with 50% reaching the blastocyst stage after fertilization (
16). In contrast to studies on mouse oocytes, only 11.1% of the porcine oocytes re-injected with porcine NLB and 13.8% with mouse NLB, were able to reach to the blastocyst stage. This group of intraspecies NLB transferred embryos shows the adverse effect of the micromanipulation procedure accompanied with the potential loss of nucleolar material To explain the low developmental capability of porcine nucleolus re-injected embryos found in this study, we decided to examine the exact timing of rDNA transcription and investigate the levels of unprocessed 45S rRNA and processed 18S rRNA during embryogenesis up to the blastocyst stage. qPCR analysis of 45S rRNA expression rate showed that the timing of genome activation in the embryos derived from porcine oocytes re-injected with porcine or mouse NLBs corresponds with the control group (8-cell stage), but the levels of transcripts are lower. These results also correspond with the timing of MGA in porcine parthenogenetic embryos detected in our previous study (
12). These results are supported by experiments with mouse enucleolated oocytes re-injected with two porcine NLBs which were able to activate rRNA synthesis at the 4-cell stage, with slightly altered expression dynamics compared to the control (
7). Interestingly, when the levels of 18S rRNA were measured, the experimental intra- and intergeneric groups of embryos showed delayed initiation of pre-rRNA processing at the blastocysts stage compared with the control. In alignment with the levels of 45S rRNA, these observations correspond with well-known theories about the exact balance between processed and unprocessed rRNA transcripts in mammalian cells (
17). The increased levels of 18S rRNA in blastocysts of intergeneric embryos compared with the control and intrageneric embryos, can be explained by higher protein amounts in mouse NLBs (1.55 ng) than those in pig (0.9 ng) (
7). These mouse, proteinrich NLBs may contain higher levels of nucleolar proteins involved in the ribosome biogenesis (
2) and thus provide more intensive support of pre-rRNA processing in porcine re-injected embryos.
CONCLUSIONThe reinjection of NLBs from mouse GV oocytes into the enucleolated MII stage porcine oocytes can support embryonic development up to the blastocyst stage after its activation. Moreover, interspecies NLB transfer embryos initiated rDNA transcription at the same time as MGA in porcine parthenogenetic embryos. Despite the alterations in processing dynamics of these embryos, our results strongly point to the potential of applied NLB transfer technique in future studies focused on the improvement of mammalian oocyte quality and thus support the developmental potential of
in vitro produced embryos.
CONFLICT OF INTERESTThe authors declare that they have no known conflict of interest in the conduction of the current study.
ACKNOWLEDGMENTSThis work was supported by the Slovak Research and Development Agency under the Contract no. DS-FR-22-0003 and by the projects “EXCELLENCE in molecular aspects of the early development of vertebrates”, CZ.02.1.01/0.0/0.0/15_003/0000460 from the Operational Programme Research, Development and Education and by the Danish Council for Independent Research/Natural Sciences (FNU) 8021-00048B. It was also co-funded by the projects VEGA 1/0167/20, 1/0270/24, KEGA 038UKF-4/2021, FVMS-IPR-02 (project no. 2020-2090/4).
AUTHORS’ CONTRIBUTIONMM designed the study and concept of the analyses and wrote the manuscript. FS revised the manuscript. MB was involved in micromanipulation, data analysis and graphic design of experimental scheme. ARB contributed in interpretation of qPCR data. SS involved in collection of mouse and porcine oocytes. FPP and MD revised the manuscript. JL gave a critical review and final approval of the version to be published. All authors have revised and approved the final version of the manuscript.

 10.2478/macvetrev-2025-0011
10.2478/macvetrev-2025-0011



